Summary
Imprinting occurs in the endosperm of flowering plants. Endosperm, produced by fertilization of the central cell in the female gametophyte, is essential for embryo and seed development. Several imprinted genes play an important role in endosperm development. The mechanism of gene imprinting involves DNA methylation and histone modification. DNA methylation is actively removed at the imprinted alleles to be activated. Histone methylation mediated by the Polycomb group complex provides another layer of epigenetic regulation at the silenced alleles. Endosperm gene imprinting can be uncoupled from seed development when fertilization of the central cell is prevented. Imprinting may be a mechanism to ensure fertilization of the central cell thereby preventing parthenogenic development of the endosperm.
Introduction
Genomic imprinting is the phenomenon in which a set of genes is expressed according to their parent of origin. Imprinting occurs primarily in the placenta of mammals and in the endosperm of flowering plants. Both structures support the developing embryo, and according to the parental conflict theory [1], imprinting is implemented to allocate limited resources to the offspring over which both paternal and maternal parents are competing. A decade ago, a specific class of mutants was identified that shows fertilization-independent seed development (for review, see [2]). Later studies revealed that these mutants are impaired in the Polycomb group (PcG) complex in the endosperm [2]. This PcG complex plays a crucial role in genomic imprinting in the endosperm, and interestingly, several of its components are imprinted.
Several years ago, DNA methylation was found to be involved in the regulation of endosperm gene imprinting [3, 4]. DNA methylation is a well-known epigenetic mark often associated with gene silencing. DNA methylation is an essential factor, regulating imprinting in both plants and mammals. Recent studies revealed that imprinting is a consequence of dynamic processes of DNA methylation and demethylation, and histone modification [5••, 6•, 7•]. In this review, we discuss the recent findings towards the understanding of imprinting mechanisms at the molecular level in Arabidopsis.
Seeds – Where Imprinting Occurs
A seed is the ripened ovule in gymnosperms and angiosperms that contains the embryo. A new plant grows from the embryo under proper conditions, which again endeavors to produce seeds for the next generation. The life cycle of Spermatophyta (seed-bearing plants) therefore begins with and ends up with seeds. Gymnosperms and angiosperms differ in seed structure and fertilization processes. In gymnosperms such as cycads or conifers, seeds are not enclosed within the ovary (thus they are called naked seeds) and the fertilization process is relatively simple. The gymnosperm female gametophyte has several archegonia in the ovule. Upon fertilization, sperm cells are released from the growing pollen tube penetrating the archegonium in which the egg cell is located. The resulting zygotic embryo absorbs nutrients from the surrounding female gametophyte tissue for growth and maturation. In contrast, the angiosperm seeds contain the endosperm, a product of double fertilization that is a distinguishing feature of flowering plants. Upon double fertilization, two sperm are released from the pollen tube into the embryo sac - a female gametophyte in angiosperms. One sperm fertilizes the egg cell and the other fertilizes the central cell. The resulting embryo and endosperm are genetically identical except for their ploidy level: the embryo is diploid and the endosperm is triploid. The endosperm, analogous to the mammalian placenta, supports and nurtures the growing embryo as does the gymnosperm female gametophyte. In the endosperm, specialized transfer cells facilitate nutrient uptake [2]. And, surprisingly, the endosperm in a developing seed is the only place where imprinting is known to occur.
Polycomb Group Genes Control Endosperm Development and are Imprinted
In angiosperms, double fertilization initiates two organs – embryo and endosperm – and their development is highly coordinated. Crosstalk between these two organs and fertilization signals appear to ensure synchronized development of each organ residing in the same ovule. However, mutations in a specific class of genes disrupt such developmental synchrony and seeds eventually abort. The Arabidopsis FIS class genes – MEDEA (MEA), FERTILIZATION-INDEPENDENT SEED2 (FIS2), FERTILIZATION-INDEPENDENT ENDOSPERM (FIE) – encode PcG components and their mutations allow the unfertilized central cell to proliferate autonomously without fertilization forming an endosperm-like structure [8, 9, 10, 11]. The characteristic seed abortion phenotype is observed only when the mutation is maternally inherited. Paternal mutations do not affect seed development.
Several imprinted genes have been identified in maize and Arabidopsis (Table 1). We discuss here the mechanisms and imprinted genes revealed in Arabidopsis and recommend to readers the following reviews [12] and [13] on maize genomic imprinting.
Table 1.
List of imprinted genes in flowering plants.
| Gene | Product | Functiona | Allelic expressionb | References |
|---|---|---|---|---|
| Arabidopsis thaliana | ||||
| FERTILIZATION-INDEPENDENT SEED2 (FIS2) | Zinc-finger transcription factor | PcG silencing | Maternal | 8, 16•, 21, 34 |
| FWA | Homeodomain transcription factor | Unknown | Maternal | 16•, 17, 35 |
| MEDEA (MEA) | PcG SET-domain protein | PcG silencing/H3K27 methylation | Maternal | 4, 5••, 6•, 7•, 9, 11, 21, 22, 26, 28•, 34, 36, 37 |
| PHERES1 (PHE1) | MADS-box transcription factor | Unknown | Paternal | 26, 27, 28• |
| Zea mays | ||||
| fertilization-independent endosperm1 (fie1) | WD-40 repeat protein | Unknown | Maternal | 38, 39, 40•, 41 |
| fertilization-independent endosperm2 (fie2) | WD-40 repeat protein | Unknown | Maternal | 38, 39, 40• |
| maternally expressed gene1 (meg1) | Cys-rich glycosylated protein | Structural role in basal endosperm transfer region (?) | Maternal | 42 |
| maize E(z)-like gene1 (mez1) | PcG SET-domain protein | Unknown | Maternal | 43 |
| no-apical-meristem-related protein (nrp) | NAM family transcription factor | Unknown | Maternal | 44 |
| Paternally expressed gene1 (peg1) | Unknown | Unknown | Paternal | 39 |
Function known in the endosperm.
Allelic expression pattern observed in the endosperm.
DNA Methylation and Demethylation in Gene Imprinting
Arabidopsis METHYLTRANSFERASE 1 (MET1), the homolog of mammalian Dnmt1, is the primary DNA methyltransferase that maintains cytosine methylation at CG sites [14]. met1 mutants display a global reduction of cytosine methylation accompanied with developmental abnormalities [15]. From genetic studies, it was shown that imprinting of MEA, FIS2, and the FWA transcription factor gene, involves MET1-mediated DNA methylation [4, 16•, 17]. Further studies revealed that there exists differential DNA methylation between the paternal and maternal alleles of MEA, FIS2, and FWA [5••, 16•]. Maternal alleles of these imprinted genes are hypomethylated, whereas the paternal alleles are hypermethylated in the endosperm. Therefore, it was hypothesized that the differential expression activity between the two parental alleles was determined by the status of DNA methylation that has been epigenetically inherited from the gametes. Unlike mammals, however, the maternal-specific expression of imprinted genes is not achieved by paternal-specific de novo methylation during gametogenesis. Rather, the default state of these imprinted genes is more likely to be MET1-dependent methylation and transcriptional silencing. Thus, a maternal-specific activator(s) releases the default silencing and activates maternal expression only in the female gametophyte. In the male gametophyte, by contrast, the paternal allele would remain silent due to an absence of a maternal-specific activator(s).
What is the maternal-specific activator(s) in the female gametophyte? Does DNA methylation serve as a silencing mark and is it removed directly or indirectly by the activator(s)? DEMETER (DME) has been identified as a transcriptional activator positively regulating MEA in the central cell [3]. DME is a parent-of-origin effect gene because only the maternal DME is important for seed viability. DME expression is confined to the central cell and its expression disappears after fertilization, whereas maternal MEA allele expression persists in the endosperm. Ovules carrying mutant dme do not express MEA and as a result the seeds eventually abort. The finding that the met1 mutation suppresses dme seed abortion by restoring MEA expression suggests DME and MET1 antagonistically regulate MEA [4]. It was thus hypothesized that DME removes DNA methylation at the maternal MEA allele in the central cell and the hypomethylated maternal MEA is exclusively expressed in the early endosperm while the methylated paternal MEA is transcriptionally silenced (Figure 1).
Figure 1. Gene imprinting during Arabidopsis seed development.
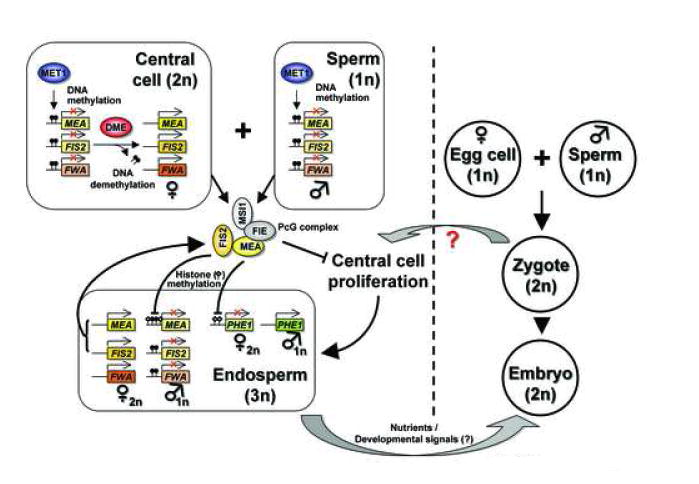
Both maternal and paternal alleles of imprinted genes are methylated by MET1 as a default state in the central cell and sperm, respectively. DME, 5-methylcytosine DNA glycosylase in the central cell, demethylates and activates MEA, FIS2, and FWA alleles [5••, 16•]. Upon fertilization, maternally expressed but paternally silenced MEA and FIS2 participate in a PcG complex. In turn, the PcG complex represses its targets such as the paternal MEA and the maternal PHE1 through histone modifications involving H3K27 methylation [5••, 28•]. Maternal MEA and FIS2, which are not repressed by the PcG complex, are continuously expressed replenishing the PcG complex, which forms an auto-regulatory feedback loop [5••]. Silencing of the paternal FIS2 and FWA appears to be solely dependent upon DNA methylation, which is inherited from the gametes [16•]. Consequently, MEA, FIS2, and FWA are maternally expressed and PHE1 paternally expressed in the early endosperm.
A recent study demonstrated that DME is necessary for demethylation and transcriptional activation of the maternal MEA in the endosperm [5••]. DME encodes a DNA glycosylase that specifically removes 5-methylcytosine from DNA [5••, 18]. DNA glycosylases are repair enzymes that initiate the base excision repair by removing damaged or mismatched bases [19]. DNA glycosylase activity of DME is required for removal of cytosine methylation both in vivo and in vitro [5••, 20]. Only the paternal MEA is methylated and silenced in the wild type endosperm, whereas, both parental alleles are methylated in the dme mutant endosperm, indicating maternal allele-specific hypomethylation [5••]. This finding suggests a mechanism of active DNA demethylation because expression of DME and its demethylation take place in the mature central cell after cell divisions within the female gametophyte have ceased. Thus, DME demethylation does not likely involve a passive demethylation process via a series of cell division without maintenance of DNA methylation. In vitro, DME removes 5-methylcytosine at any sequence contexts, whereas in vivo DME demethylation occurs in a locus-specific manner [5••]. Moreover, such DNA demethylation activity is observed at specific sites even within the same locus. For example, regulation of DNA methylation and demethylation at the maternal MEA only takes place in the 5’ and 3’ of the coding region [5••]. How DME is targeted to a specific region is still unknown. DME is also required for maternal activation of two other imprinted genes FIS2 and FWA, and their DNA methylation/demethylation pattern in both parental alleles is very similar to that of MEA [16•, 17].
Therefore, imprinting of MEA, FIS2, and FWA in the endosperm is initiated and established by DME-mediated active DNA demethylation in the central cell, while the paternal alleles remain methylated and silenced (Figure 1). The methylation state of each allele is likely to persist via epigenetic mechanisms throughout nuclear divisions during early endosperm development. The on/off switch of DNA methylation is sufficient for the establishment and maintenance of both FIS2 and FWA imprinting [16•]. By contrast, MEA imprinting requires an additional regulatory mechanism, which is discussed below.
Maintenance of Gene Imprinting by PcG Silencing
Both MEA and FIS2 are imprinted in the endosperm. MEA is homologous to Drosophila E(z) whose SET domain has methyltransferase activity on lysine 27 of histone 3 (H3K27) [9, 11]. FIS2 is a zinc-finger transcription factor homologous to Drosophila Suppressor of Zeste12 [Su(z)12] [21]. The FIS class gene products, MEA, FIS2, and FIE appear to function in a large PcG complex along with additional components such as MULTI-COPY SUPPRESSOR OF IRA1 (MSI1) and retinoblastoma-related protein RBR1[22, 23, 24]. This multimeric PcG complex is predicted to repress gene transcription via histone modification and chromatin remodeling, and the established patterns are stably propagated through mitotic cell cycles [25]. This PcG complex is thought to negatively regulate endosperm cell proliferation because autonomous central cell divisions occur in mea, fis2, or fie mutants in the absence of fertilization [8, 9, 10, 11].
Activation by demethylation of the maternal MEA allele is accomplished by DME in the central cell [3, 5••], while the paternal allele is methylated and silenced. The differential methylation patterns are inherited in the endosperm after fertilization. However, DNA methylation is not directly involved in the maintenance of paternal MEA silencing because even the unmethylated paternal MEA allele contributed by met1 mutants is not expressed in the endosperm [5••]. Rather, the FIS-PcG complex containing MEA itself seems to keep the silenced paternal MEA repressed [5••, 6•, 7•]. Disruption of the FIS-PcG complex causes loss of MEA imprinting as silencing of the paternal allele is released [5••, 6•, 7•]. In addition, MEA is physically associated with the MEA promoter sequence [7•]. These findings propose a self-imprinting mechanism of MEA, in which maternally expressed MEA replenishes the FIS-PcG complex, and in turn, the complex keeps repressing the silenced paternal MEA allele (Figure 1) [5••].
PHERES1 (PHE1) is another imprinted gene in the Arabidopsis endosperm [26]. Whereas MEA, FIS2, and FWA are maternally expressed, paternal PHE1 expression predominates in the endosperm, while the maternal PHE1 is silent or very weakly expressed [27]. The silenced maternal PHE1 allele is a direct target of the FIS-PcG complex [27]. In mea mutant seeds, for example, silencing of the maternal PHE1 is released leading to biallelic expression [27]. Unlike other imprinted genes, however, the role of DNA methylation in PHE1 imprinting is not reported. Rather, histone modification via the FIS-PcG complex likely both establishes and maintains the silencing of the paternal PHE1 (Figure 1).
Notably, MEA is required for H3K27 methylation, one of the epigenetic silencing marks, at the silenced paternal MEA and the maternal PHE1 alleles [5••, 6•, 28•]. Silencing of the paternal MEA is released in the mea mutant endosperm accompanied with loss of H3K27 methylation [5••]. Repression of the PHE1 allele is also associated with H3K27 methylation [28•]. A mutation in the catalytic center of the MEA SET domain abolishes PHE1 repression, suggesting that histone methyltransferase activity of MEA is necessary for its function in PcG silencing and gene imprinting [28•].
Imprinting Bypass and Seed Development
Genomic imprinting in the Arabidopsis endosperm is regulated by both DNA methylation and PcG silencing. Is imprinting an integral feature of seed development that cannot be uncoupled? A recent study demonstrated that seeds are produced without double fertilization by bypassing genomic imprinting [29••]. Mutants for CDKA;1 which encodes a Cdc2/Cdc28 homologue produce pollen with only one sperm [30•, 31•]. This mutant pollen preferentially fertilizes the egg cell while the binucleate central cell remains unfertilized. Embryos from eggs fertilized with cdka;1 mutant pollen abort about 3 days after pollination and only a few divisions of the unfertilized central cell occurs [31•]. This suggests that a positive signal is generated from a developing embryo to initiate central cell proliferation independent of second fertilization. Strikingly, disruption of PcG inhibition in the female gametophyte allows single-fertilized seeds with unfertilized homodiploid endosperm [29••]. When PcG mutants such as mea, fis2, and fie are pollinated with cdka;1 pollen, viable seeds form albeit the seed size is smaller than wild type [29••]. This implies that genomic imprinting in the endosperm is not necessary for seed development under certain circumstances and that an unfertilized diploid central cell in the female gametophyte has the full potential to develop functional endosperm without a paternal contribution. These results support the hypothesis that during the evolution of plants, the multicellular gymnosperm female gametophyte was reduced to the central cell in the angiosperm female gametophyte, and that fertilization of the central cell is the trigger that activates development of the multicellular endosperm. [29••].
Conclusions
Two epigenetic mechanisms, DNA methylation and histone modification involving PcG proteins, regulate gene imprinting in seed development. Initiation of gene imprinting requires DME in the female gametophyte for allele-specific DNA demethylation. Differential methylation distinguishes the two parental alleles in the endosperm after fertilization and results in parent-of-origin patterns of gene expression. These allele-specific epigenetic marks are maintained and fortified by the PcG complex, which, in turn, auto-regulates its own components. Such epigenetic regulation and imprinting are vital to proper endosperm development and seed viability since mutations in the components of this regulatory circuit produce unviable seeds.
Nevertheless, loss of imprinting (i.e., gain of biallelic expression) does not always compromise seed development. When the paternal genome is derived from met1 mutants, FIS2 and FWA are biparentally expressed in the endosperm producing viable seeds [16•]. Fertilization of a fis mutant ovule with cdka;1 pollen produces viable seeds with homodiploid endosperm in the absence of paternal genome contribution, thus bypassing the requirement of gene imprinting [29••]. That the diploid condition is sufficient for a viable seed is evident by the presence of biparental diploid endosperm in Nuphar polysepalum, a basal angiosperm [32]. Therefore it is reasonable to speculate that endosperms of most flowering plants might have evolved a unique imprinting mechanism to ensure that fertilization of the central cell takes place, and that the male contributes to the production of healthy endosperm for the next generation. Thus, in flowering plants and mammals, imprinting prevents parthenogenic development of the endosperm and embryo, respectively [33].
Acknowledgments
We acknowledge support from the National Institutes of Health (GM069415), United States Department of Agriculture (2005-02355), and the United States - Israel Binational Research Fund (IS-3604-04CR) to Robert L. Fischer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haig D, Wilczek A. Sexual conflict and the alternation of haploid and diploid generations. Philos T Roy Soc B. 2006;361:335–343. doi: 10.1098/rstb.2005.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gehring M, Choi Y, Fischer RL. Imprinting and seed development. Plant Cell. 2004;16:S203–S213. doi: 10.1105/tpc.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi YH, Gehring M, Johnson L, Hannon M, Harada JJ, Goldberg RB, Jacobsen SE, Fischer RL. DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell. 2002;110:33–42. doi: 10.1016/s0092-8674(02)00807-3. [DOI] [PubMed] [Google Scholar]
- 4.Xiao WY, Gehring M, Choi Y, Margossian L, Pu H, Harada JJ, Goldberg RB, Pennell RI, Fischer RL. Imprinting of the MEA polycomb gene is controlled by antagonism between MET1 methyltransferase and DME glycosylase. Dev Cell. 2003;5:891–901. doi: 10.1016/s1534-5807(03)00361-7. [DOI] [PubMed] [Google Scholar]
- 5••.Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. DME 5-methylcytosine DNA glycosylase plays a role in active DNA demethylation. Differential DNA methylation levels between the MEA parental alleles are responsible for gene imprinting in the endosperm. This study also revealed allele-specific histone methylation of MEA, proposing a mechanism of self-imprinting.
- 6•.Jullien PE, Katz A, Oliva M, Ohad N, Berger F. Polycomb group complexes self-regulate imprinting of the polycomb group gene MEDEA in Arabidopsis. Curr Biol. 2006;16:486–492. doi: 10.1016/j.cub.2006.01.020. This study uses chromatin immunoprecipitation (ChIP) to show that the PcG complex, containing MEA, aids the silencing of the paternal MEA allele by H3K27 methylation. Thus, MEA is involved in its own regulation and imprinting by maintaining paternal allele silencing through chromatin modifications.
- 7•.Baroux U, Gagliardini V, Page DR, Grossniklaus U. Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Gene Dev. 2006;20:1081–1086. doi: 10.1101/gad.378106. By using ChIP, this study reveals that MEA can independently bind to its own promoter without the aid of other PcG complex proteins. This provides further evidence that MEA is autoregulating its own expression. The authors also show that the PcG complex, either directly or indirectly, is involved in stimulating expression at some loci.
- 8.Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ. Fertilization-independent seed development in Arabidopsis thaliana. P Natl Acad Sci USA. 1997;94:4223–4228. doi: 10.1073/pnas.94.8.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by medea, a Polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- 10.Ohad N, Margossian L, Hsu YC, Williams C, Repetti P, Fischer RL. A mutation that allows endosperm development without fertilization. P Natl Acad Sci USA. 1996;93:5319–5324. doi: 10.1073/pnas.93.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiyosue T, Ohad N, Yadegari R, Hannon M, Dinneny J, Wells D, Katz A, Margossian L, Harada JJ, Goldberg RB, et al. Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. P Natl Acad Sci USA. 1999;96:4186–4191. doi: 10.1073/pnas.96.7.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott RJ, Spielman M. Deeper into the maize: new insights into genomic imprinting in plants. Bioessays. 2006;28:1167–1171. doi: 10.1002/bies.20508. [DOI] [PubMed] [Google Scholar]
- 13.Penterman J, Huh JH, Hsieh TF, Fischer RL. Genomic Imprinting in Arabidopsis thaliana and Zea mays. Plant Cell Monogr. 2007;8:219–239. [Google Scholar]
- 14.Finnegan EJ, Dennis ES. Isolation and identification by sequence homology of a putative cytosine methyltransferase from Arabidopsis thaliana. Nucleic Acids Res. 1993;21:2383–2388. doi: 10.1093/nar/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnegan EJ, Peacock WJ, Dennis ES. Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. P Natl Acad Sci USA. 1996;93:8449–8454. doi: 10.1073/pnas.93.16.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jullien PE, Kinoshita T, Ohad N, Berger F. Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell. 2006;18:1360–1372. doi: 10.1105/tpc.106.041178. FIS2 and FWA imprinting requires the activity of MET1 in both the central cell and pollen. Abolishing MET1 expression in the pollen does not affect MEA imprinting.
- 17.Kinoshita T, Miura A, Choi YH, Kinoshita Y, Cao XF, Jacobsen SE, Fischer RL, Kakutani T. One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science. 2004;303:521–523. doi: 10.1126/science.1089835. [DOI] [PubMed] [Google Scholar]
- 18.Morales-Ruiz T, Ortega-Galisteo AP, Ponferrada-Marin MI, Martinez-Macias MI, Ariza RR, Roldan-Arjona T. DEMETER and REPRESSOR OFSILENCING 1 encode 5-methylcytosine DNA glycosylases. P Natl Acad Sci USA. 2006;103:6853–6858. doi: 10.1073/pnas.0601109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharer OD, Jiricny J. Recent progress in the biology, chemistry and structural biology of DNA glycosylases. Bioessays. 2001;23:270–281. doi: 10.1002/1521-1878(200103)23:3<270::AID-BIES1037>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 20.Choi Y, Harada JJ, Goldberg RB, Fischer RL. An invariant aspartic acid in the DNA glycosylase domain of DEMETER is necessary for transcriptional activation of the imprinted MEDEA gene. P Natl Acad Sci USA. 2004;101:7481–7486. doi: 10.1073/pnas.0402328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM. Genes controlling fertilization-independent seed development in Arabidopsis thaliana. P Natl Acad Sci USA. 1999;96:296–301. doi: 10.1073/pnas.96.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadegari R, Kinoshita T, Lotan O, Cohen G, Katz A, Choi Y, Katz A, Nakashima K, Harada JJ, Goldberg RB, et al. Mutations in the FIE and MEA genes that encode interacting polycomb proteins cause parent-of-origin effects on seed development by distinct mechanisms. Plant Cell. 2000;12:2367–2381. doi: 10.1105/tpc.12.12.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohler C, Hennig L, Bouveret R, Gheyselinck J, Grossniklaus U, Gruissem W. Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. Embo J. 2003;22:4804–4814. doi: 10.1093/emboj/cdg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebel C, Mariconti L, Gruissem W. Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature. 2004;429:776–780. doi: 10.1038/nature02637. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh TF, Fischer RL. Biology of chromatin dynamics. Annu Rev Plant Biol. 2005;56:327–351. doi: 10.1146/annurev.arplant.56.032604.144118. [DOI] [PubMed] [Google Scholar]
- 26.Kohler C, Hennig L, Spillane C, Pien S, Gruissem W, Grossniklaus U. The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Gene Dev. 2003;17:1540–1553. doi: 10.1101/gad.257403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler C, Page DR, Gagliardini V, Grossniklaus U. The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet. 2005;37:28–30. doi: 10.1038/ng1495. [DOI] [PubMed] [Google Scholar]
- 28•.Makarevich G, Leroy O, Akinci U, Schubert D, Clarenz O, Goodrich J, Grossniklaus U, Kohler C. Different Polycomb group complexes regulate common target genes in Arabidopsis. Embo Rep. 2006;7:947–952. doi: 10.1038/sj.embor.7400760. The SET domain of MEA catalyzes H3K27 methylation at the PHE1 allele in the endosperm. PHE1 is also targeted by different PcG complexes in vegetative tissues. These suggest that different PcG complexes may target the same genes during different stages of plant development.
- 29••.Nowack MK, Shirzadi R, Dissmeyer N, Dolf A, Endl E, Grini PE, Schnittger A. Bypassing genomic imprinting allows seed development. Nature. 2007;447:312–U313. doi: 10.1038/nature05770. This study shows that viable seeds can be developed by bypassing genomic imprinting in the endosperm. Bypassing is allowed only when the female gametophyte is mutant for the PcG genes. Fertilization of PcG mutant ovule with cdka;1 pollen [31•] triggers the central cell to proliferate and make functional homodiploid endosperm without the second fertilization.
- 30•.Iwakawa H, Shinmyo A, Sekine M. Arabidopsis CDKA; 1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 2006;45:819–831. doi: 10.1111/j.1365-313X.2005.02643.x. This study describes a loss of function mutation in CDKA;1. This mutation prevents second pollen mitosis forming a mature pollen grain that contains one vegetative cell and one sperm cell that is still capable of fertilizing the egg. They also describe the phenotypic consequences of ovules that are fertilized by this pollen grain.
- 31•.Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A. A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet. 2006;38:63–67. doi: 10.1038/ng1694. This paper was the first to describe the failure of the second pollen mitosis in cdka;1 mutants. The authors further showed that the unfertilized central cell goes through a few rounds of nuclear divisions, suggesting that a signal is delivered from the fertilized embryo to trigger development of the endosperm, independently of the second fertilization.
- 32.Williams JH, Friedman WE. Identification of diploid endosperm in an early angiosperm lineage. Nature. 2002;415:522–526. doi: 10.1038/415522a. [DOI] [PubMed] [Google Scholar]
- 33.Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, Park ES, Seo JS, Ogawa H. Birth of parthenogenetic mice that can develop to adulthood. Nature. 2004;428:860–864. doi: 10.1038/nature02402. [DOI] [PubMed] [Google Scholar]
- 34.Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A. Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. P Natl Acad Sci USA. 2000;97:10637–10642. doi: 10.1073/pnas.170292997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soppe WJJ, Jacobsen SE, Alonso-Blanco C, Jackson JP, Kakutani T, Koornneef M, Peeters AJM. The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol Cell. 2000;6:791–802. doi: 10.1016/s1097-2765(05)00090-0. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita T, Yadegari R, Harada JJ, Goldberg RB, Fischer RL. Imprinting of the MEDEA polycomb gene in the Arabidopsis endosperm. Plant Cell. 1999;11:1945–1952. doi: 10.1105/tpc.11.10.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vielle-Calzada JP, Thomas J, Spillane C, Coluccio A, Hoeppner MA, Grossniklaus U. Maintenance of genomic imprinting at the Arabidopsis medea locus requires zygotic DDM1 activity. Gene Dev. 1999;13:2971–2982. doi: 10.1101/gad.13.22.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danilevskaya ON, Hermon P, Hantke S, Muszynski MG, Kollipara K, Ananiev EV. Duplicated fie genes in maize: Expression pattern and imprinting suggest distinct functions. Plant Cell. 2003;15:425–438. doi: 10.1105/tpc.006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez-Marcos JF, Pennington PD, Costa LM, Dickinson HG. Imprinting in the endosperm: a possible role in preventing wide hybridization. Philos T Roy Soc B. 2003;358:1105–1111. doi: 10.1098/rstb.2003.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Gutierrez-Marcos JF, Costa LM, Dal Pra M, Scholten S, Kranz E, Perez P, Dickinson HG. Epigenetic asymmetry of imprinted genes in plant gametes. Nat Genet. 2006;38:876–878. doi: 10.1038/ng1828. In maize gene imprinting, gametogenesis establishes asymmetric DNA methylation between the paternal and maternal alleles, which marks them as distinct in the endosperm after fertilization. This study also revealed the existence of de novo DNA methylation at the paternal FIE2 allele for its silencing after fertilization.
- 41.Hermon P, Srilunchang KO, Zou J, Dresselhaus T, Danilevskaya ON. Activation of the imprinted Polycomb Group Fie1 gene in maize endosperm requires demethylation of the maternal allele. Plant Mol Biol. 2007;64:387–395. doi: 10.1007/s11103-007-9160-0. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez-Marcos JF, Costa LM, Biderre-Petit C, Khbaya B, O’Sullivan DM, Wormald M, Perez P, Dickinson HG. maternally expressed gene1 is a novel maize endosperm transfer cell-specific gene with a maternal parent-of-origin pattern of expression. Plant Cell. 2004;16:1288–1301. doi: 10.1105/tpc.019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haun WJ, Laoueillé-Duprat S, O’connell MJ, Spillane C, Grossniklaus U, Phillips AR, Kaeppler SM, Springer NM. Genomic imprinting, methylation and molecular evolution of maize Enhancer of zeste (Mez) homologs. Plant J. 2007;49:325–337. doi: 10.1111/j.1365-313X.2006.02965.x. [DOI] [PubMed] [Google Scholar]
- 44.Guo M, Rupe MA, Danilevskaya ON, Yang XF, Hut ZH. Genome-wide mRNA profiling reveals heterochronic allelic variation and a new imprinted gene in hybrid maize endosperm. Plant J. 2003;36:30–44. doi: 10.1046/j.1365-313x.2003.01852.x. [DOI] [PubMed] [Google Scholar]


