Abstract
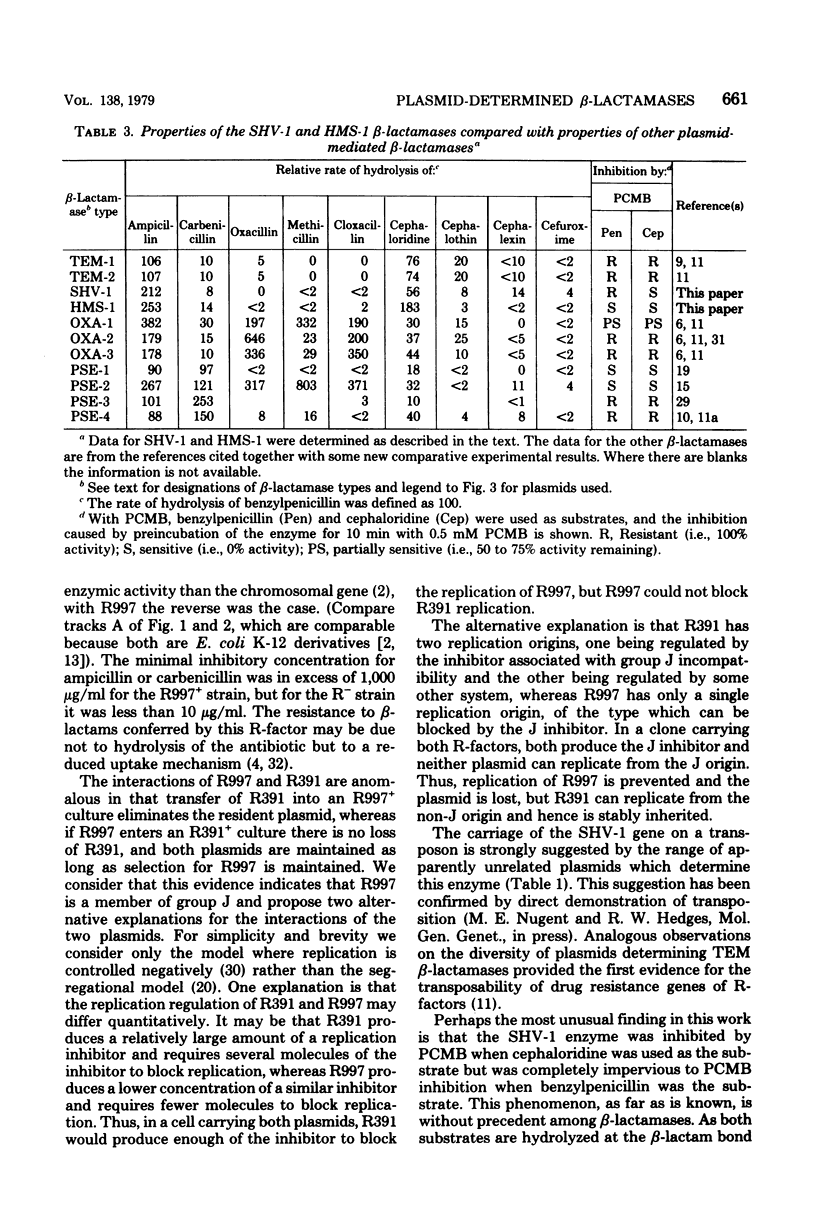
Two species of beta-lactamase determined by plasmids in enteric bacteria that show some resemblance to TEM enzymes are described. Both are distinct from all other plasmid-mediated beta-lactamases and differ from the TEM beta-lactamases in ability to hydrolyze some substrates, in isoelectric point, in immunological specificity, and in susceptibility to inhibition. One of the enzyme species, mediated by plasmid p453, has been briefly described previously. We have discovered that this beta-lactamase, designated SHV-1, is unique in its response to inhibition by the sulfhydryl group reagent p-chloromercuribenzoate, because the hydrolysis of cephaloridine but not that of benzylpenicillin is affected. This enzyme is found in a variety of plasmid types which were transferred from several bacterial species collected from a wide geographic range. The other enzyme species is novel; only a single plasmid determining this kind of beta-lactamase (designated HMS-1) has been detected.
Full text
PDF

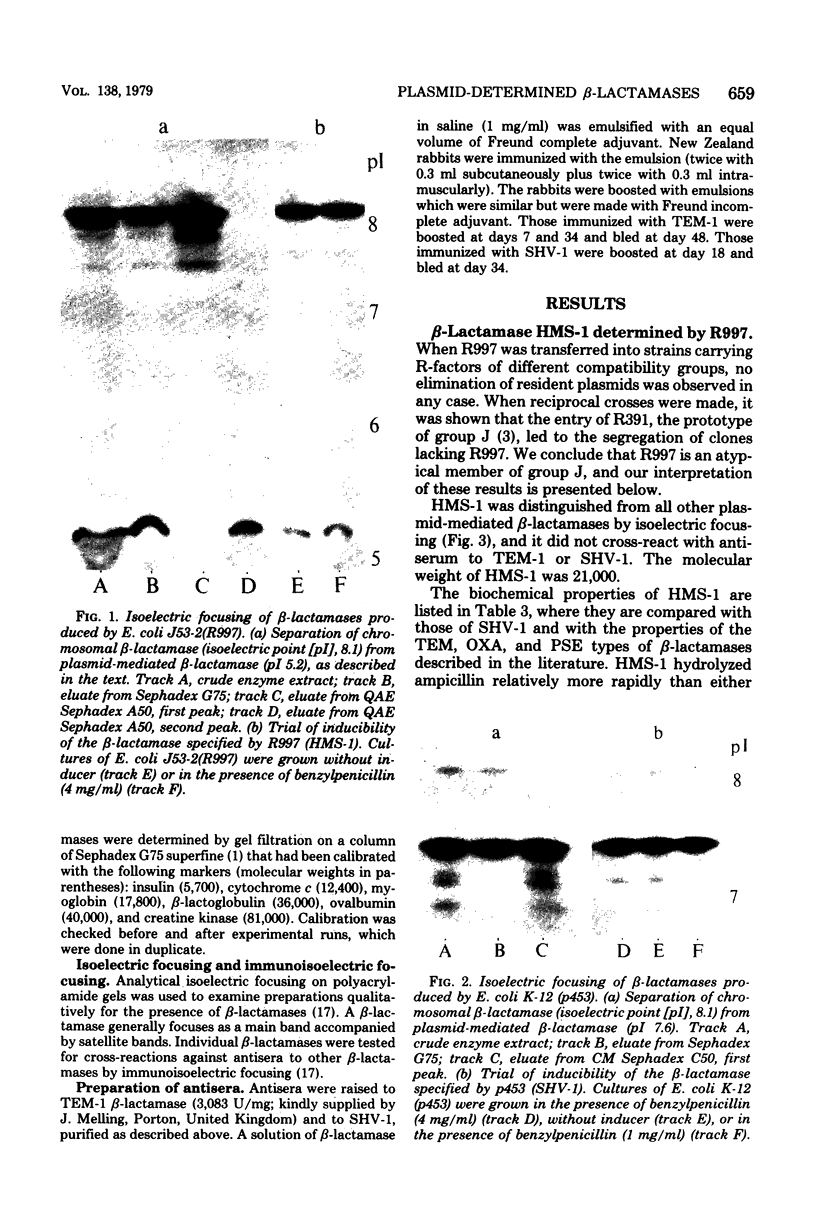


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrowski M. M., Matthew M., Barth P. T., Datta N., Grinter N. J., Jacob A. E., Kontomichalou P., Dale J. W., Smith J. T. Plasmid-determined beta-lactamase indistinguishable from the chromosomal beta-lactamase of Escherichia coli. J Bacteriol. 1976 Jan;125(1):149–157. doi: 10.1128/jb.125.1.149-157.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Richmond M. H. Effect of R-factor-mediated genes on some surface properties of Escherichia coli. Antimicrob Agents Chemother. 1974 Dec;6(6):666–671. doi: 10.1128/aac.6.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. R-factor-mediated beta-lactamases that hydrolyze oxacillin: evidence for two distinct groups. J Bacteriol. 1974 Aug;119(2):351–356. doi: 10.1128/jb.119.2.351-356.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The purification and properties of the -lactamase specified by the resistance factor R-1818 in Escherichia coli and Proteus mirabilis. Biochem J. 1971 Jul;123(4):493–500. doi: 10.1042/bj1230493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison S. Naturally occurring R factor, derepressed for pilus synthesis, belonging to the same compatibility group as the sex factor F of Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):416–422. doi: 10.1128/jb.109.1.416-422.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth A. J. Purification and properties of a constitutive beta-lactamase from Pseudomonas aeruginosa strain Dalgleish. Biochim Biophys Acta. 1975 Feb 19;377(2):431–443. doi: 10.1016/0005-2744(75)90323-x. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Matthew M. Acquisition by Escherichia coli of plasmid-borne beta-lactamases normally confined to Pseudomonas spp. Plasmid. 1979 Apr;2(2):269–278. doi: 10.1016/0147-619x(79)90045-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labia R., Le Goffic F., Andrillon J. Etude cinétique de deux beta lactamases responsables d'un møeme phénotype. Biochimie. 1974;56(8):1025–1030. doi: 10.1016/s0300-9084(74)80092-1. [DOI] [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Matthew M., Harris A. M. Identification of beta-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J Gen Microbiol. 1976 May;94(1):55–67. doi: 10.1099/00221287-94-1-55. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Sykes R. B. Properties of the beta-lactamase specified by the Pseudomonas plasmid RPL11. J Bacteriol. 1977 Oct;132(1):341–345. doi: 10.1128/jb.132.1.341-345.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Hoppensteadt F. C. On plasmid incompatibility. Plasmid. 1978 Sep;1(4):421–434. doi: 10.1016/0147-619x(78)90001-x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Petrocheilou V., Sykes R. B., Richmond M. H. Novel R-plasmid-mediated beta-lactamase from Klebsiella aerogenes. Antimicrob Agents Chemother. 1977 Jul;12(1):126–128. doi: 10.1128/aac.12.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A., Paul G., Labia R., Nevot P. Distinction entre les beta-lactamases immunotypes 1 et 2 de Pitton grâce à une nouvelle beta-lactamine. Ann Microbiol (Paris) 1976 May-Jun;127(4):487–491. [PubMed] [Google Scholar]
- Pitton J. S. Mechanisms of bacterial resistance to antibiotics. Ergeb Physiol. 1972;65:15–93. doi: 10.1007/3-540-05814-1_2. [DOI] [PubMed] [Google Scholar]
- Roupas A., Pitton J. S. R factor-mediated and chromosomal resistance to ampicillin in Escherichia coli. Antimicrob Agents Chemother. 1974 Feb;5(2):186–191. doi: 10.1128/aac.5.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. Plasmid incompatibility and control of replication: copy mutants of the R-factor R1 in Escherichia coli K-12. J Bacteriol. 1975 Nov;124(2):641–649. doi: 10.1128/jb.124.2.641-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma S., Terakado N., Mitsuhashi S. Biochemical properties of a penicillin beta-lactamase mediated by R factor from Bordetella bronchiseptica. Antimicrob Agents Chemother. 1975 Sep;8(3):238–242. doi: 10.1128/aac.8.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Beta-lactamase-directed barrier for penicillins of Escherichia coli carrying R plasmids. Antimicrob Agents Chemother. 1977 Jun;11(6):936–940. doi: 10.1128/aac.11.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]