Abstract
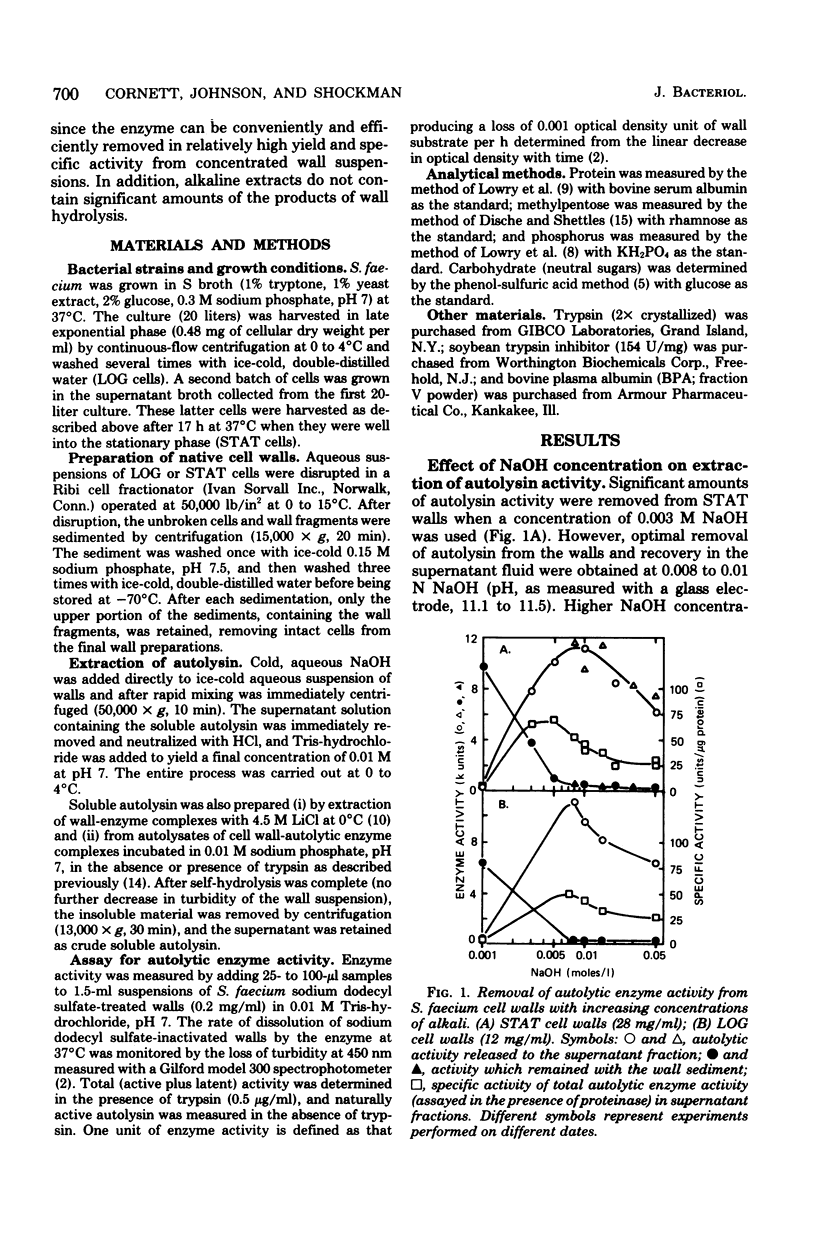
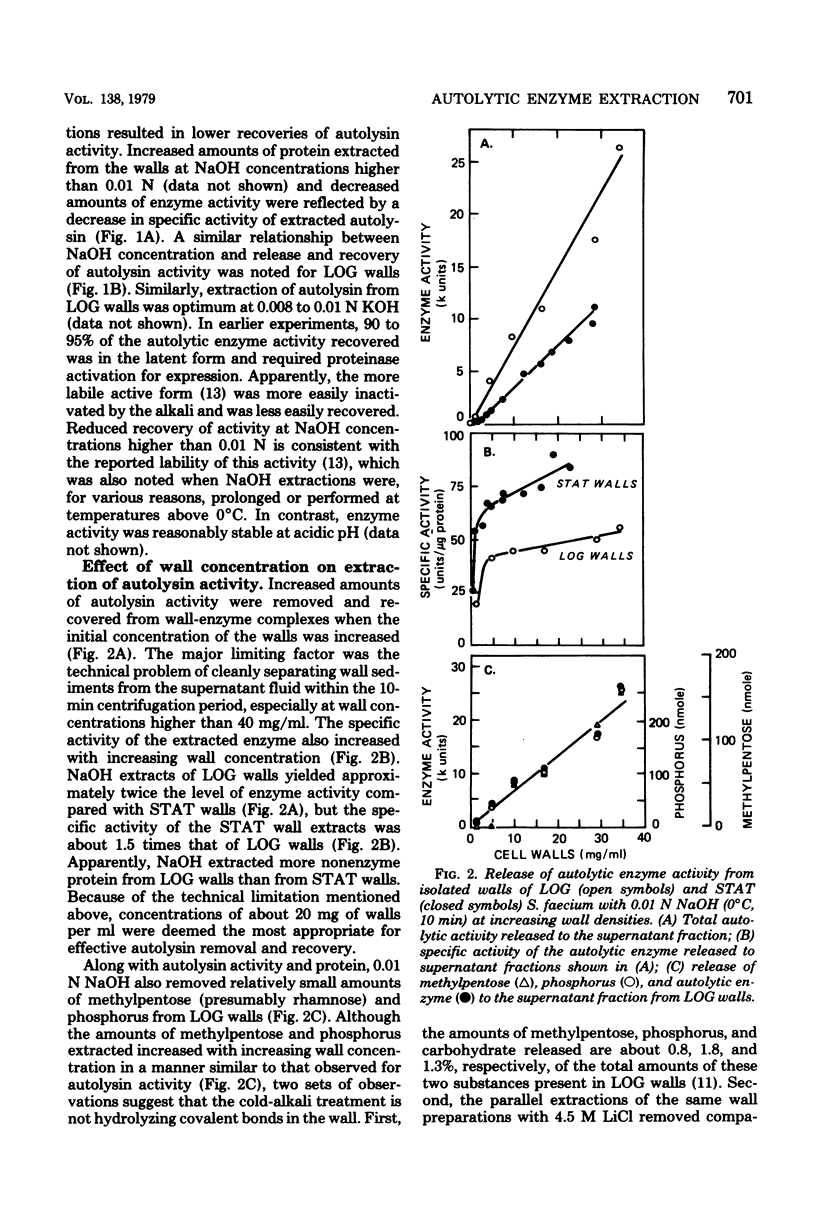
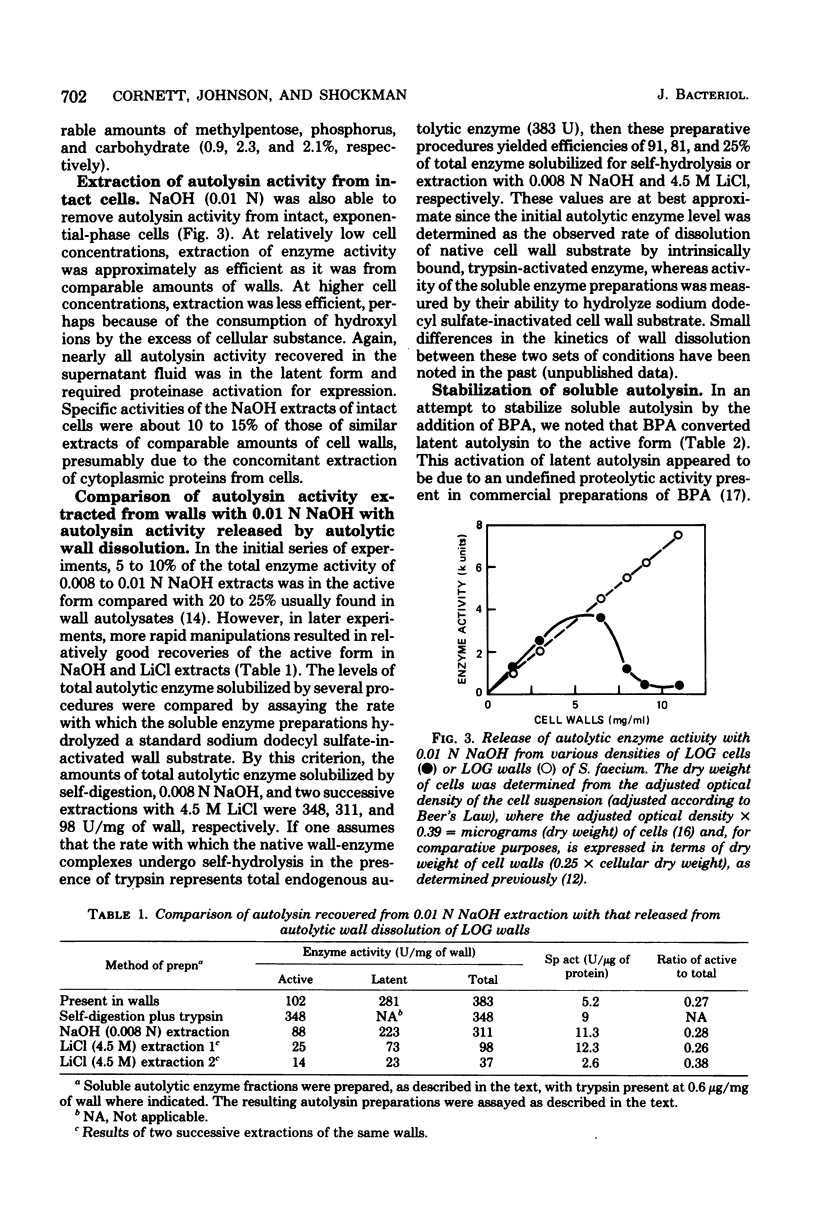
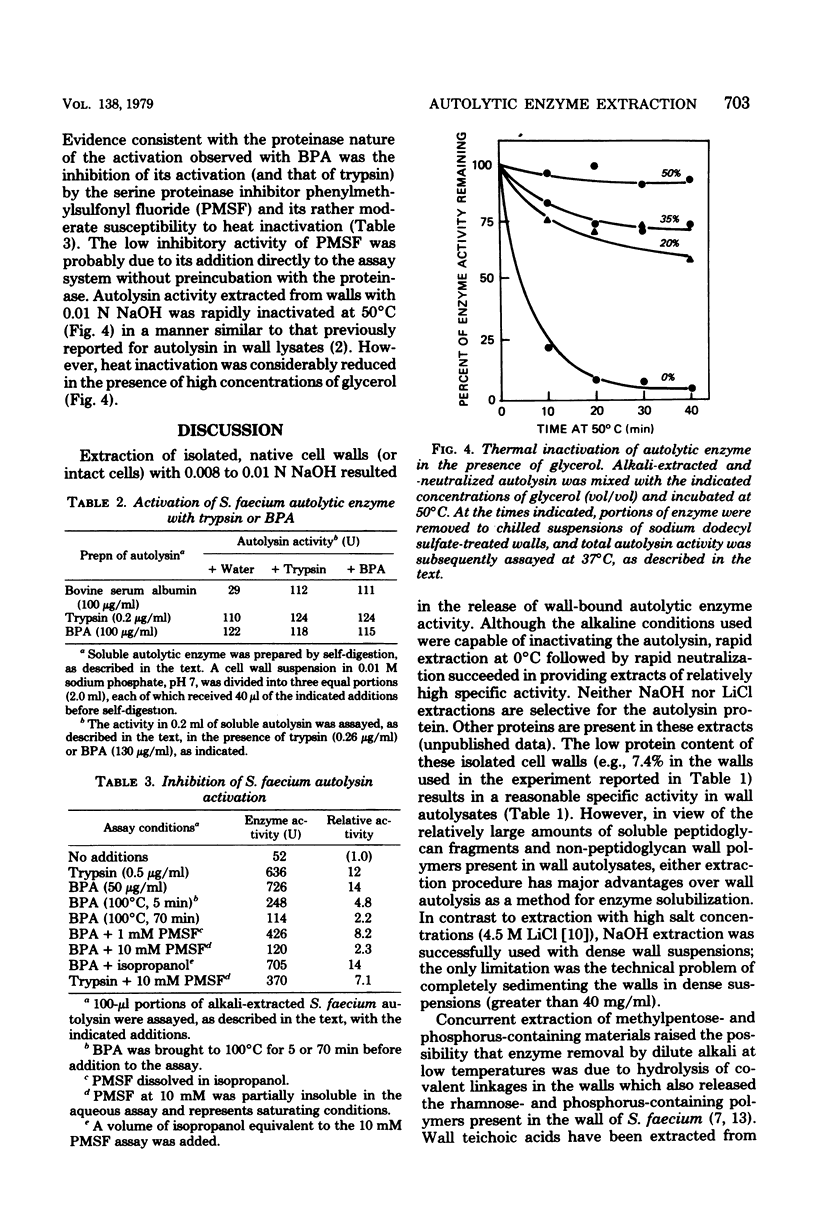
The autolytic enzyme (endo-beta-1,4-N-acetylmuramoylhydrolase) of Streptococcus faecium (S. faecalis ATCC 9790) was released in a soluble form from insoluble cell wall-autolytic enzyme complexes by treatment with dilute NaOH at 0 degree C. Treatment of cell wall-enzyme complexes, obtained from either exponential- or stationary-phase cells, with 0.008 to 0.01 N NaOH gave maximum yields of autolytic enzyme activity. At a fixed concentration of NaOH, the yield of autolysin increased with increasing wall densities and was accompanied by the release of methylpentose and phosphorus in amounts proportional to the autolysin. Since extraction of wall-enzyme complexes with 4.5 M LiCl at 0 degree C also removed methylpentose and phosphorus, release of enzyme with NaOH did not appear to result from hydrolysis of covalent linkages. The autolytic enzyme activity released from intact cells, or cell walls, was predominantly in the later (proteinase activable) form which could be activated by trypsin or a proteinase present in commerical bovine plasma albumin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. C., Fraser D. K., Young F. E. Problems in purification of a Bacillus subtilis autolytic enzyme caused by association with teichoic acid. Biochim Biophys Acta. 1970 Feb 11;198(2):308–315. doi: 10.1016/0005-2744(70)90063-x. [DOI] [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Shockman G. D. Some properties of the autolytic N-acetylmuramidase of Lactobacillus acidophilus. J Bacteriol. 1973 Apr;114(1):34–41. doi: 10.1128/jb.114.1.34-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J. The action of dilute alkali on some bacterial cell walls. Biochem Biophys Res Commun. 1968 Oct 10;33(1):22–28. doi: 10.1016/0006-291x(68)90248-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pooley H. M., Porres-Juan J. M., Shockman G. D. Dissociation of an autolytic enzyme-cell wall complex by treatment with unusually high concentrations of salt. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1134–1140. doi: 10.1016/0006-291x(70)90357-8. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D., Higgins M. L., Porres-Juan J. Some properties of two autolytic-defective mutants of Streptococcus faecalis ATCC 9790. J Bacteriol. 1972 Jan;109(1):423–431. doi: 10.1128/jb.109.1.423-431.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D. Bacterial cell wall synthesis: the effect of threonine depletion. J Biol Chem. 1959 Sep;234:2340–2342. [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Foster J. F. Conformation-dependent limited proteolysis of bovine plasma albumin by an enzyme present in commercial albumin preparations. Biochemistry. 1971 May 11;10(10):1772–1780. doi: 10.1021/bi00786a007. [DOI] [PubMed] [Google Scholar]


