Abstract
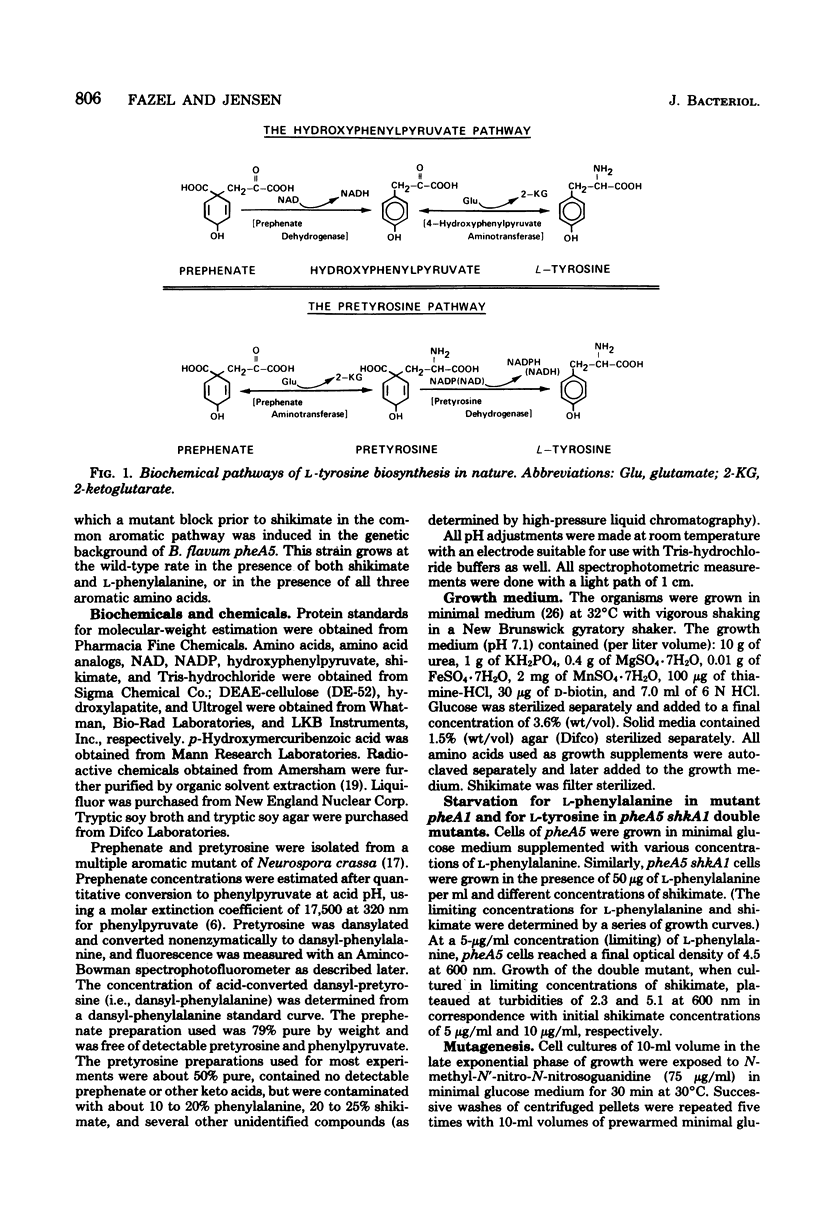
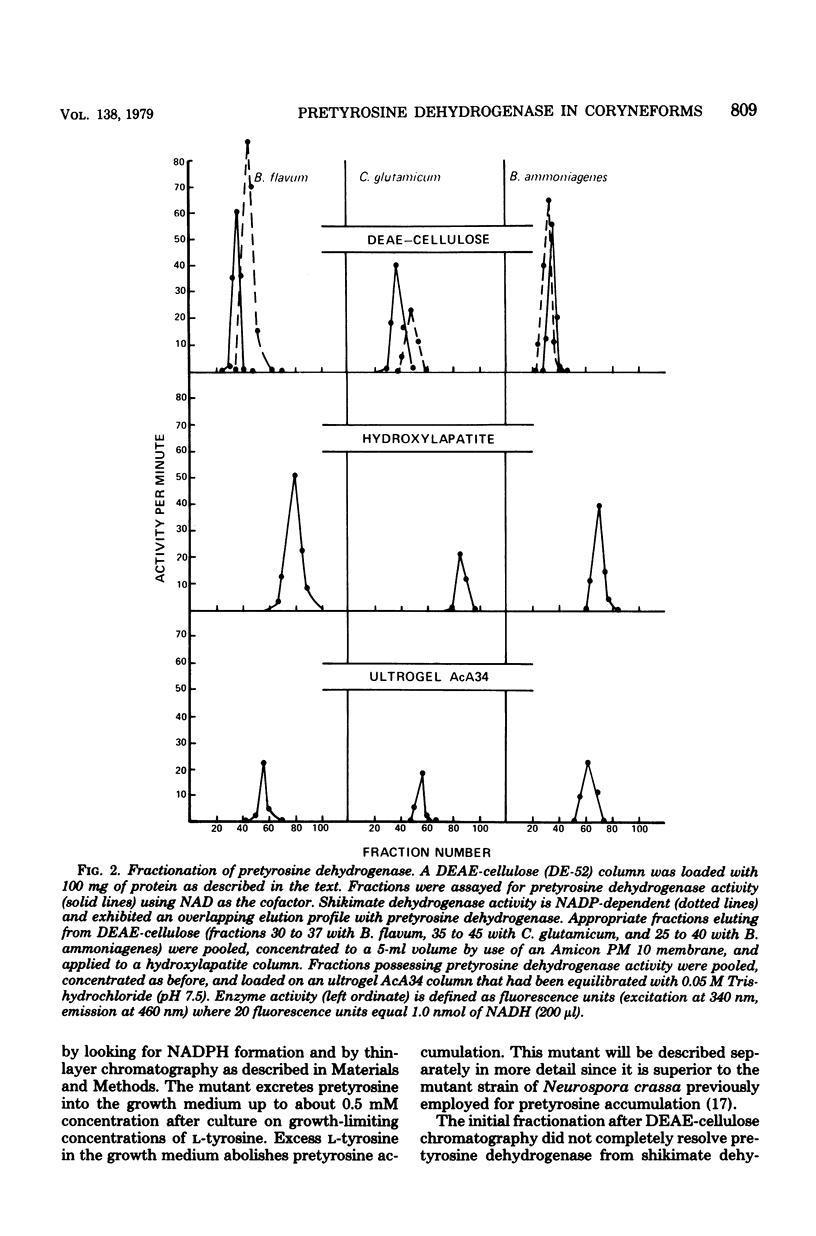
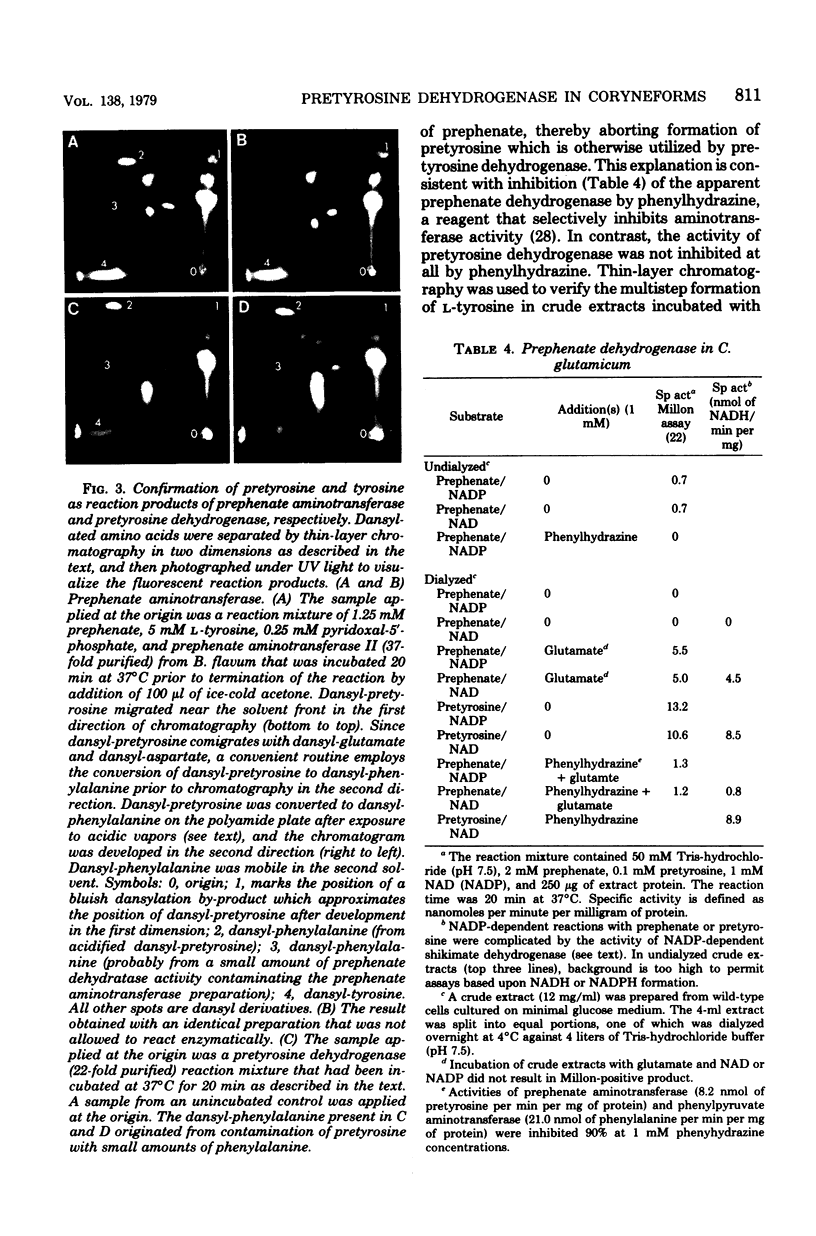
Species of coryneform bacteria (Corynebacterium glutamicum, Brevibacterium flavum, and B. ammoniagenes) utilize pretyrosine [beta-(1-carboxy-4-hydroxy-2,5-cyclohexadien-1-yl) alanine] as an intermediate in L-tyrosine biosynthesis. Pretyrosine is formed from prephenate via the activity of at least one species of aromatic aminotransferase which is significantly greater with prephenate as substrate than with either phenylpyruvate or 4-hydroxyphenylpyruvate. Pretyrosine dehydrogenase, capable of converting pretyrosine to L-tyrosine, has been partially purified from all three species. Each of the three pretyrosine dehydrogenases is catalytically active with either nicotinamide adenine dinucleotide or nicotinamide adenine dinucleotide phosphate as cofactors. The Km values for nicotinamide adenine dinucleotide phosphate in C. glutamicum and B. flavum are 55 microM and 14.2 microM, respectively, and corresponding Km values for nicotinamide adenine dinucleotide are 350 microM and 625 microM, respectively. The molecular weights of pretyrosine dehydrogenase in C. glutamicum and in B. flavum are both about 158,000, compared with 68,000 moleculr weitht in B. ammoniagenes. In all three species the enzyme is not feedback inhibited by L-tyrosine. Results obtained with various auxotropic mutants, which were used to manipulate internal concentrations of L-tyrosine, suggest that pretyrosine dehydrogenase is expressed constitutively. Pretyrosine dehydrogenase is quite sensitive to p-hydroxymercuribenzoic acid, complete inhibition being achieved at 10 to 25 microM concentrations. This inhibition is readily reversed by thiol reagents such as 2-mercaptoethanol. Coryneform organisms, like species of blue-green bacteria, appear to lack the 4-hydroxyphenylpyruvate pa thway of L-tyrosine synthesis altogether. The loss of pretyrosine dehydrogenase in extracts prepared from a tyrosine auxotroph affirms the exclusive role of pretyrosine dehydrogenase in L-tyrosine biosynthesis. Other reports in the literature, in which the presence in these organisms of prephenate dehydrogenase is described, appear to be erroneous.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Champney W. S., Jensen R. A. The enzymology of prephenate dehydrogenase in Bacillus subtilis. J Biol Chem. 1970 Aug 10;245(15):3763–3770. [PubMed] [Google Scholar]
- Cohen G. N., Stanier R. Y., Le Bras G. Regulation of the biosynthesis of amino acids of the aspartate family in Coliform bacteria and Pseudomonads. J Bacteriol. 1969 Sep;99(3):791–801. doi: 10.1128/jb.99.3.791-801.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Friedrich C. G., Schlegel H. G. Purification and properties of chorismate mutase-prephenate dehydratase and prephenate dehydrogenase from Alcaligenes eutrophus. J Bacteriol. 1976 May;126(2):712–722. doi: 10.1128/jb.126.2.712-722.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Schlegel H. G. Aromatic amino acid biosynthesis in Alcaligenes eutrophus H16. II. The isolation and characterization of mutants auxotrophic for phenylalanine and tyrosine. Arch Microbiol. 1975 Apr 7;103(2):141–149. doi: 10.1007/BF00436341. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Enzyme recruitment in evolution of new function. Annu Rev Microbiol. 1976;30:409–425. doi: 10.1146/annurev.mi.30.100176.002205. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Pierson D. L. Evolutionary implications of different types of microbial enzymology for L-tyrosine biosynthesis. Nature. 1975 Apr 24;254(5502):667–671. doi: 10.1038/254667a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. A. Taxonomic implications of temperature dependence of the allosteric inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase in Bacillus. J Bacteriol. 1970 May;102(2):489–497. doi: 10.1128/jb.102.2.489-497.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. L., Shaw D. C., Gibson F. The purification and characterisation of chorismate mutase-prephenate dehydrogenase from Escherichia coli K12. Biochim Biophys Acta. 1971 Mar 23;229(3):795–804. doi: 10.1016/0005-2795(71)90298-4. [DOI] [PubMed] [Google Scholar]
- Miller J. V., Jr, Thompson E. B. Radioactive assay for tyrosine aminotransferase. Anal Biochem. 1972 Jun;47(2):487–494. doi: 10.1016/0003-2697(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Patel N., Stenmark-Cox S. L., Jensen R. A. Enzymological basis of reluctant auxotrophy for phenylalanine and tyrosine in Pseudomonas aeruginosa. J Biol Chem. 1978 May 10;253(9):2972–2978. [PubMed] [Google Scholar]
- SCHWINCK I., ADAMS E. Aromatic biosynthesis. XVI. Aromatization of prephenic acid to p-hydroxyphenylpyruvic acid, a step in tyrosine biosynthesis in Escherichia coli. Biochim Biophys Acta. 1959 Nov;36:102–117. doi: 10.1016/0006-3002(59)90074-5. [DOI] [PubMed] [Google Scholar]
- Shiio I., Sugimoto S., Miyajima R. Regulation of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthetase in Brevibacterium flavum. J Biochem. 1974 May;75(5):987–997. doi: 10.1093/oxfordjournals.jbchem.a130501. [DOI] [PubMed] [Google Scholar]
- Stenmark S. L., Pierson D. L., Jensen R. A., Glover G. I. Blue-green bacteria synthesise L-tyrosine by the pretyrosine pathway. Nature. 1974 Feb 1;247(5439):290–292. doi: 10.1038/247290a0. [DOI] [PubMed] [Google Scholar]
- Veldkamp H. Saprophytic coryneform bacteria. Annu Rev Microbiol. 1970;24:209–240. doi: 10.1146/annurev.mi.24.100170.001233. [DOI] [PubMed] [Google Scholar]