Abstract
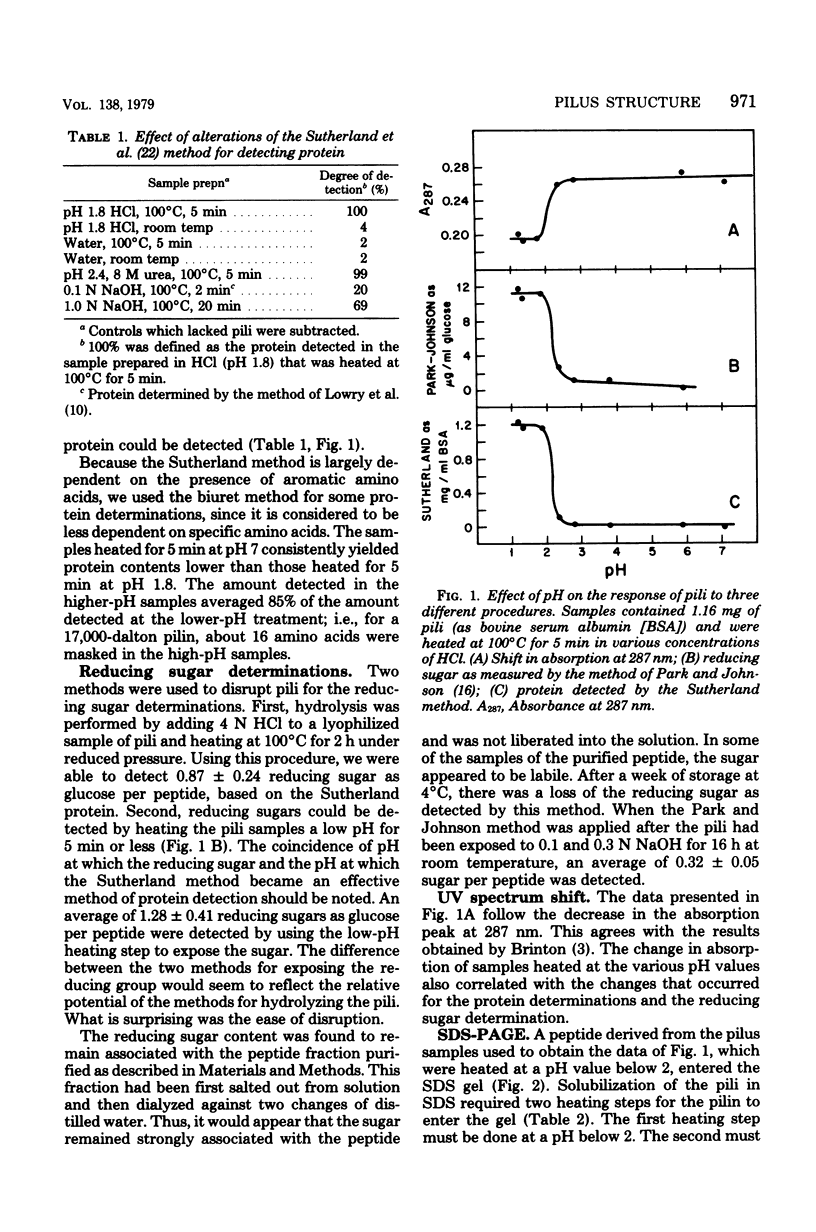
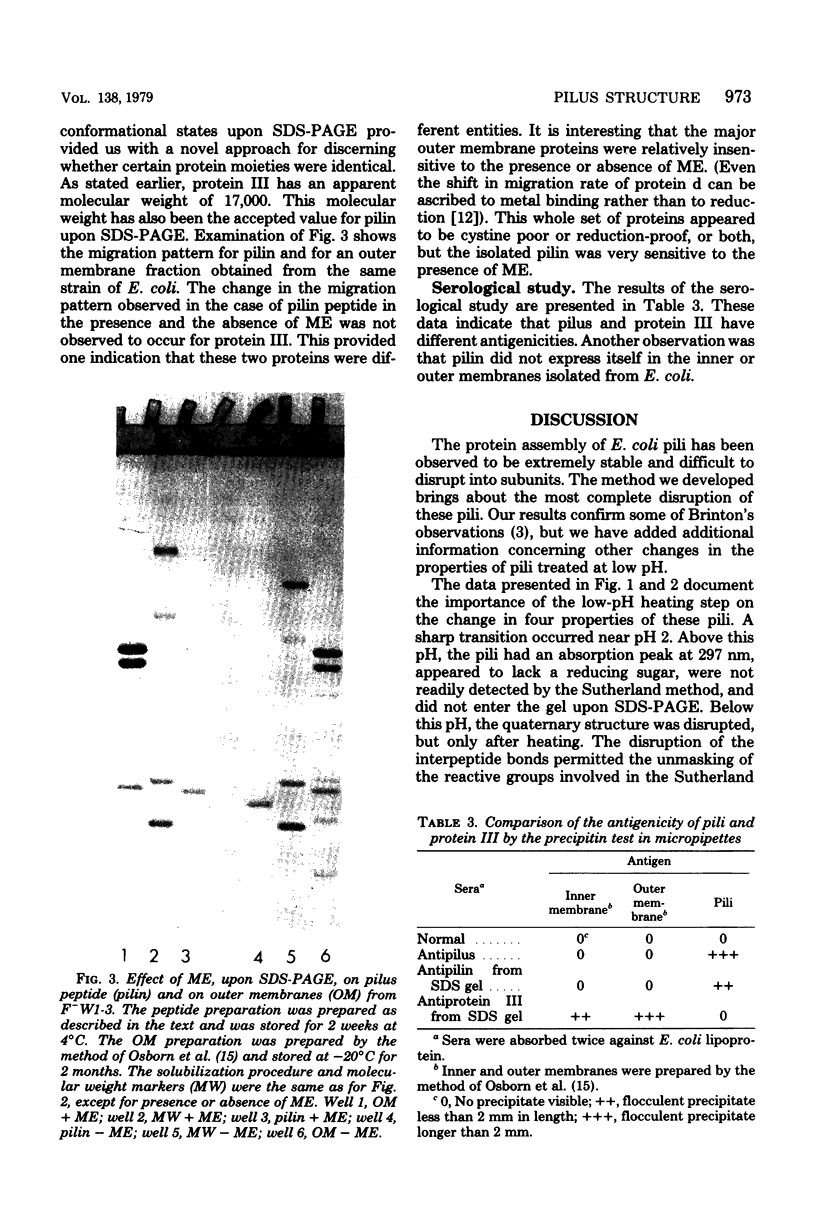
Several important properties of the common pili from Escherichia coli are discussed. These pili were resistant to the gentle Folin-Ciocalteau reagent methods for protein detection and were not readily solubilized by sodium dodecyl sulfate. They were found to contain a reducing sugar but not peptidoglycan. The pilin had multiple conformations in sodium dodecyl sulfate solution, and the appearance of multiple bands on sodium dodecyl sulfate gels did not necessarily indicate heterogeneity of the preparation. The ilus subunit was found to be a different protein than outer membrane III, which has the same apparent molecular weight. In addition, we conformed the results of Brinton (Trans. N.Y. Acad. Sci 27:1003-1054, 1965): that there is a dramatic change in the properties of pili after they are heated at pH values below 2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, BUZZELL A., LAUFFER M. A. Electrophoresis and phage susceptibility studies on a filament-producing variant of the E. coli B bacterium. Biochim Biophys Acta. 1954 Dec;15(4):533–542. doi: 10.1016/0006-3002(54)90011-6. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Dreesman G. R., Hollinger F. B., Suriano J. R., Fujioka R. S., Brunschwig J. P., Melnick J. L. Biophysical and biochemical heterogeneity of purified hepatitis B antigen. J Virol. 1972 Sep;10(3):469–476. doi: 10.1128/jvi.10.3.469-476.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Metal ion dependence of a heat-modifiable protein from the outer membrane of Escherichia coli upon sodium dodecyl sulfate-gel electrophoresis. J Bacteriol. 1977 Oct;132(1):314–320. doi: 10.1128/jb.132.1.314-320.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Novotny C., Carnahan J., Brinton C. C., Jr Mechanical removal of F pili, type I pili, and flagella from Hfr and RTF donor cells and the kinetics of their reappearance. J Bacteriol. 1969 Jun;98(3):1294–1306. doi: 10.1128/jb.98.3.1294-1306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]