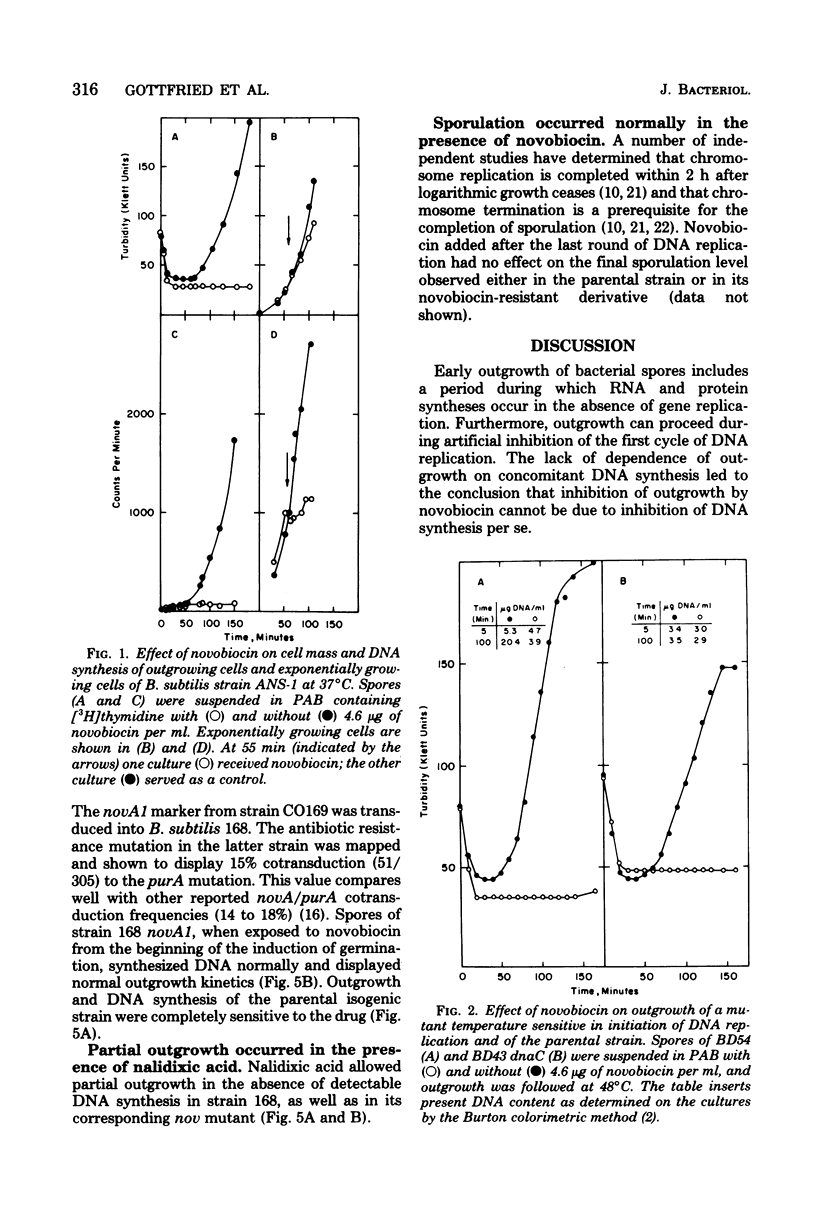
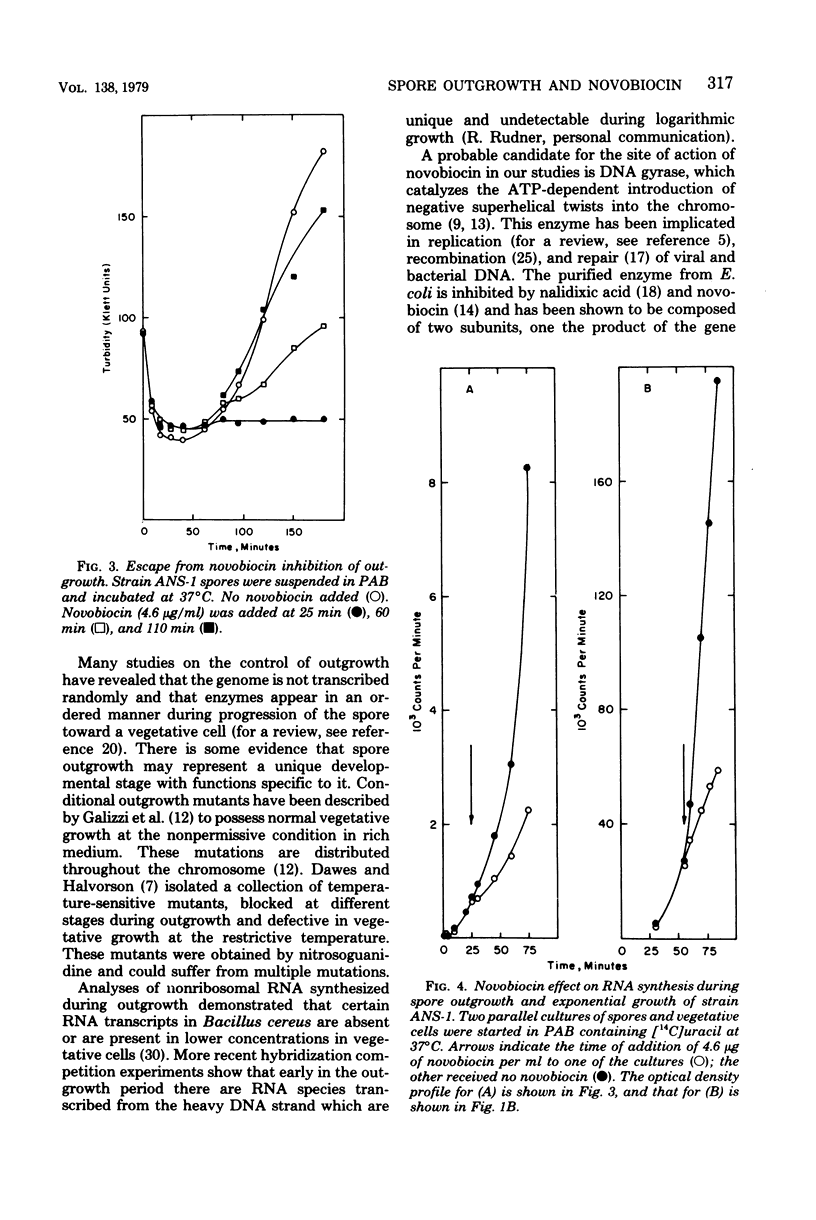
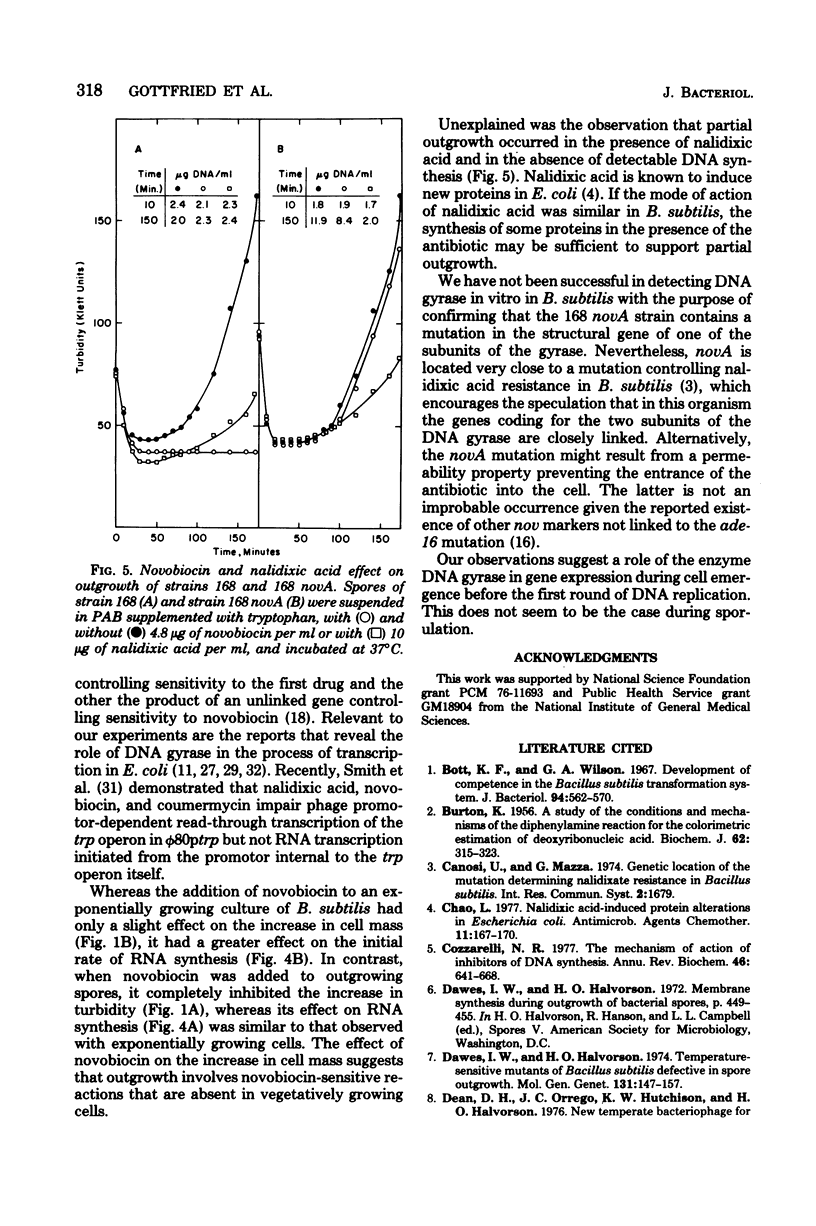
Abstract
Spores of a Bacillus subtilis mutant temperature sensitive in deoxyribonucleic acid (DNA) replication proceeded through outgrowth at the nonpermissive temperature to the same extent as the wild-type parent spores. In contrast, the DNA synthesis inhibitor novobiocin completely prevented spore outgrowth while displaying a marginal effect on logarithmic growth during one generation time. Inhibition of outgrowth by novobiocin occurred in the absence of DNA replication, as demonstrated in an experiment with spores of the temperature-sensitive DNA synthesis mutant at the restrictive temperature. Novobiocin inhibited the initial rate of ribonucleic acid synthesis to the same extent in germinated spores and in exponentially growing cells. A novobiocin-resistant mutant underwent normal outgrowth in the presence of novobiocin. Therefore, novobiocin inhibition was independent of its effect on chromosome replication per se.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L. Nalidixic acid-induced protein alterations in Escherichia coli. Antimicrob Agents Chemother. 1977 Jan;11(1):167–170. doi: 10.1128/aac.11.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Halvorson H. O. Temperature-sensitive mutants of Bacillus subtilis defective in spore outgrowth. Mol Gen Genet. 1974;131(2):147–157. doi: 10.1007/BF00266150. [DOI] [PubMed] [Google Scholar]
- Dean D. H., Orrego J. C., Hutchison K. W., Halvorson H. O. New temperate bacteriophage for Bacillus subtilis, rho 11. J Virol. 1976 Nov;20(2):509–519. doi: 10.1128/jvi.20.2.509-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Snyder M. Superhelical Escherichia coli DNA: relaxation by coumermycin. J Mol Biol. 1978 Apr 5;120(2):145–154. doi: 10.1016/0022-2836(78)90061-x. [DOI] [PubMed] [Google Scholar]
- Falco S. C., Zivin R., Rothman-Denes L. B. Novel template requirements of N4 virion RNA polymerase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3220–3224. doi: 10.1073/pnas.75.7.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., Keynan A. Independence of Bacillus subtilis spore outgrowth from DNA synthesis. J Bacteriol. 1978 Oct;136(1):111–116. doi: 10.1128/jb.136.1.111-116.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford N., Sueoka N. Chromosomal location of antibiotic resistance markers in Bacillus subtilis. J Mol Biol. 1970 Jul 28;51(2):267–286. doi: 10.1016/0022-2836(70)90142-7. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Boehmer S. Antagonists of DNA gyrase inhibit repair and recombination of UV-irradiated phage lambda. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4125–4129. doi: 10.1073/pnas.75.9.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins N. P., Peebles C. L., Sugino A., Cozzarelli N. R. Purification of subunits of Escherichia coli DNA gyrase and reconstitution of enzymatic activity. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1773–1777. doi: 10.1073/pnas.75.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamata D., Gross J. D. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol Gen Genet. 1970;108(3):277–287. doi: 10.1007/BF00283358. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Sterlini J. M., Kay D. Sporulation in Bacillus subtilis. Effect of medium on the form of chromosome replication and on initiation to sporulation in Bacillus subtilis. Biochem J. 1971 Nov;125(2):635–641. doi: 10.1042/bj1250635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Gross J. D. Characterization of a temperature-sensitive mutant of Bacillus subtilis defective in deoxyribonucleic acid replication. J Bacteriol. 1967 Nov;94(5):1603–1608. doi: 10.1128/jb.94.5.1603-1608.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Nash H. A. Restriction assay for integrative recombination of bacteriophage lambda DNA in vitro: requirement for closed circular DNA substrate. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3524–3528. doi: 10.1073/pnas.73.10.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego C., Arnaud M., Halvorsen H. O. Bacillus subtilis 168 genetic transformation mediated by outgrowing spores: necessity for cell contact. J Bacteriol. 1978 Jun;134(3):973–981. doi: 10.1128/jb.134.3.973-981.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Tessman I. Mechanism of transcription of bacteriophage S13. II. Inhibition of phage-specific transcription by nalidixic acid. J Mol Biol. 1973 Mar 25;75(1):99–108. doi: 10.1016/0022-2836(73)90531-7. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Halvorson H. O. Nature of deoxyribonucleic acid synthesis and its relationship to protein synthesis during outgrowth of Bacillus cereus T. J Bacteriol. 1972 Feb;109(2):606–615. doi: 10.1128/jb.109.2.606-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H., Schwartz M. The effect of nalidixic acid on the expression of some genes in Escherichia coli K-12. Biochem Biophys Res Commun. 1975 May 5;64(1):204–209. doi: 10.1016/0006-291x(75)90239-9. [DOI] [PubMed] [Google Scholar]
- Silberstein Z., Cohen A. Hybridization analysis of restriction endonuclease DNA fragments of Bacillus cereus transcribed during spore outgrowth. J Bacteriol. 1978 Jun;134(3):1081–1088. doi: 10.1128/jb.134.3.1081-1088.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Kubo M., Imamoto F. Promoter-specific inhibition of transcription by antibiotics which act on DNA gyrase. Nature. 1978 Oct 5;275(5679):420–423. doi: 10.1038/275420a0. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. Replication of Escherichia coli DNA in vitro: inhibition by oxolinic acid. Eur J Biochem. 1976 Mar 1;62(3):491–497. doi: 10.1111/j.1432-1033.1976.tb10183.x. [DOI] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. II. Relationship between ordered enzyme synthesis and deoxyribonucleic acid replication. J Bacteriol. 1968 Feb;95(2):479–489. doi: 10.1128/jb.95.2.479-489.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Doi R. H. Differential expression of bacteriophage genomes in vegetative and sporulating cells of Bacillus subtilis. J Virol. 1967 Oct;1(5):935–947. doi: 10.1128/jvi.1.5.935-947.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


