Abstract
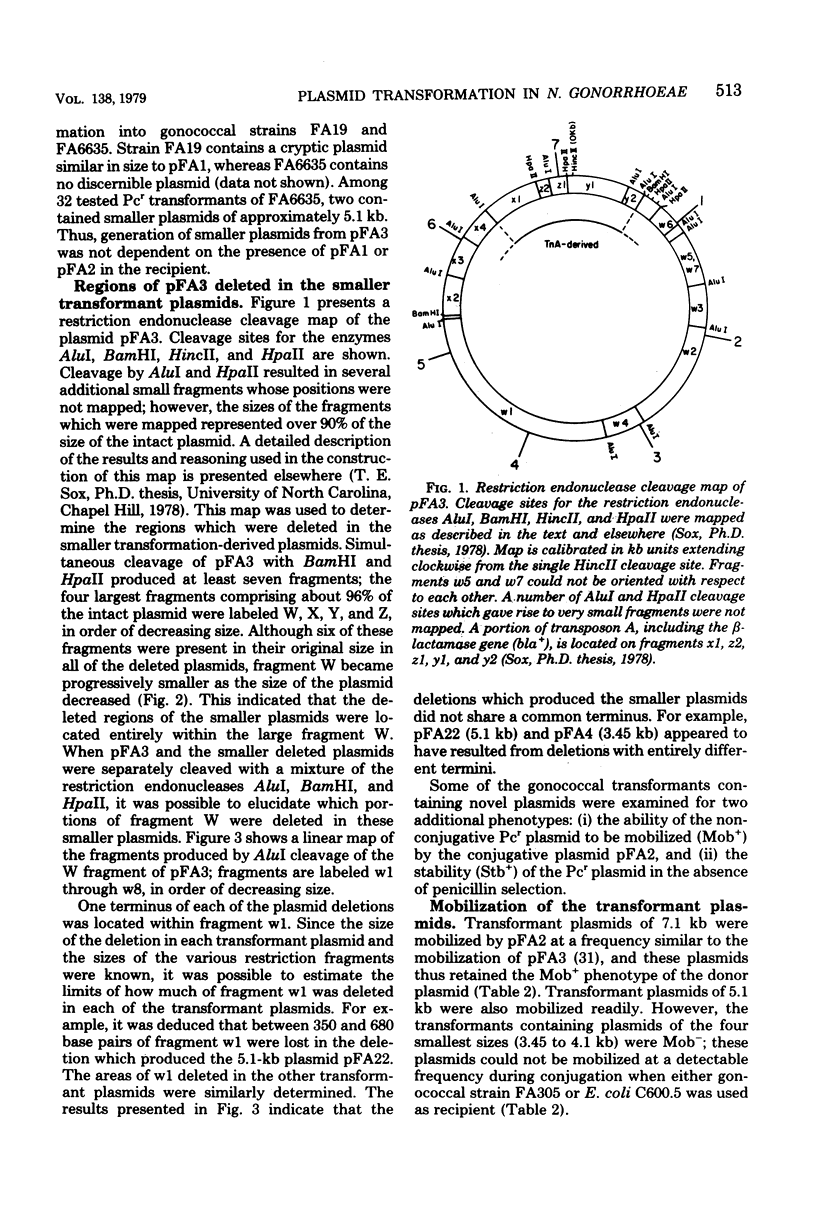
Plasmid deoxyribonucleic acid from Neisseria gonorrhoeae containing a 7.1-kilobase (kb) (4.7-megadalton) penicillinase (Pcr) plasmid transformed homogenic gonococci to penicillinase production at a low frequency. About 25% of the penicillinase-producing gonococcal transformants contained Pcr plasmids which were either larger or smaller than the 7.1 kb donor plasmid; these Pcr plasmids varied in size from 3.45 to 42 kb. Some of these altered plasmids differed from the donor plasmid in stability or in frequency of mobilization by a 36-kb (24-megadalton) conjugative plasmid. A restriction endonuclease cleavage map of the 7.1-kilobase Pcr plasmid and several of the smaller deleted plasmids was constructed. The most common size of altered Pcr plasmid was 5.1 kb (3.4 megadaltons). A Pcr plasmid isolated from a gonococcus in London, England, was identical with these 5.1-kb transformant plasmids in both size and restriction endonuclease cleavage profiles, suggesting that the 5.1-kb Pcr plasmid could have arisen from a 7.1-kb Pcr plasmid by a transformation-associated deletion in nature.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Natkin E. Transduction of resistance determinants and R factors of the transfer systems by phage Plkc. Mol Gen Genet. 1972;114(3):261–265. doi: 10.1007/BF01788895. [DOI] [PubMed] [Google Scholar]
- Bernhard K., Schrempf H., Goebel W. Bacteriocin and antibiotic resistance plasmids in Bacillus cereus and Bacillus subtilis. J Bacteriol. 1978 Feb;133(2):897–903. doi: 10.1128/jb.133.2.897-903.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Sox T., Blackman E., Sparling P. F. Factors affecting genetic transformation of Neisseria gonorrhoeae. J Bacteriol. 1977 Feb;129(2):983–992. doi: 10.1128/jb.129.2.983-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G., Comer S., Sparling P. F. Chromosomal location of antibiotic resistance genes in Neisseria gonorrhoeae. J Bacteriol. 1976 Mar;125(3):1207–1210. doi: 10.1128/jb.125.3.1207-1210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Mylroie J. R., Friello D. A., Vacca J. G. Transformation of Pseudomonas putida and Escherichia coli with plasmid-linked drug-resistance factor DNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3647–3651. doi: 10.1073/pnas.72.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Sox T., Biswas G., Blackman E., Sparling P. F. Conjugal transfer of the gonococcal penicillinase plasmid. Science. 1977 Mar 11;195(4282):998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- Goebel W., Lindenmaier W., Pfeifer F., Schrempf H., Schelle B. Transposition and insertion of intact, deleted and enlarged ampicillin transposon Tn3 from mini-R1 (Rsc) plasmids into transfer factors. Mol Gen Genet. 1977 Nov 29;157(2):119–129. doi: 10.1007/BF00267389. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Dubnau D. Construction and properties of chimeric plasmids in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1428–1432. doi: 10.1073/pnas.75.3.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Studies of colicin E1 plasmid functions by analysis of deletions and TnA insertions of the plasmid. J Bacteriol. 1977 Oct;132(1):332–340. doi: 10.1128/jb.132.1.332-340.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahari K. Deletion plasmids from transformants of Pseudomonas aeruginosa trp cells with the RSF1010-trp hybrid plasmid and high levels of enzyme activity from the gene on the plasmid. J Bacteriol. 1978 Oct;136(1):312–317. doi: 10.1128/jb.136.1.312-317.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Takanami M. Cleavage map of colicin E1 plasmid. Nature. 1976 Nov 11;264(5582):193–196. doi: 10.1038/264193a0. [DOI] [PubMed] [Google Scholar]
- Phillips I. Beta-lactamase-producing, penicillin-resistant gonococcus. Lancet. 1976 Sep 25;2(7987):656–657. doi: 10.1016/s0140-6736(76)92466-1. [DOI] [PubMed] [Google Scholar]
- Roberts M., Elwell L. P., Falkow S. Molecular characterization of two beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae. J Bacteriol. 1977 Aug;131(2):557–563. doi: 10.1128/jb.131.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Falkow S. Conjugal transfer of R plasmids in Neisseria gonorrhoeae. Nature. 1977 Apr 14;266(5603):630–631. doi: 10.1038/266630a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Blackman E., Sparling P. F. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol. 1974 Dec;120(3):1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F. Transfer of antibiotic resistance in mixed cultures of Neisseria gonorrhoeae. J Infect Dis. 1974 Dec;130(6):660–663. doi: 10.1093/infdis/130.6.660. [DOI] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Sox T. E., Mohammed W., Blackman E., Biswas G., Sparling P. F. Conjugative plasmids in Neisseria gonorrhoeae. J Bacteriol. 1978 Apr;134(1):278–286. doi: 10.1128/jb.134.1.278-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M., Richmond M. H. Characterization of a translocation unit encoding resistance to mercuric ions that occurs on a nonconjugative plasmid in Pseudomonas aeruginosa. J Bacteriol. 1977 Mar;129(3):1227–1233. doi: 10.1128/jb.129.3.1227-1233.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]
- Warren G., Sherratt D. Complementation of transfer deficient ColE1 mutants. Mol Gen Genet. 1977 Mar 7;151(2):197–201. doi: 10.1007/BF00338695. [DOI] [PubMed] [Google Scholar]




