Abstract
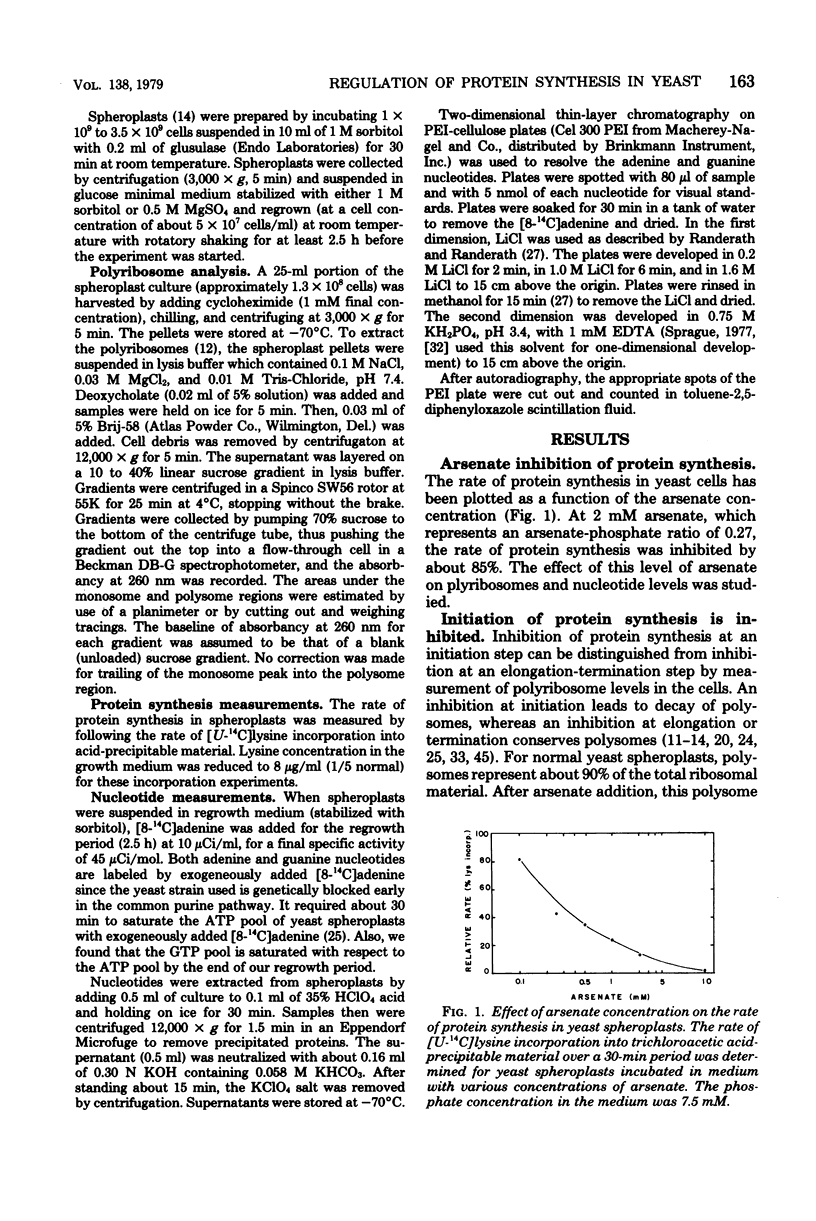
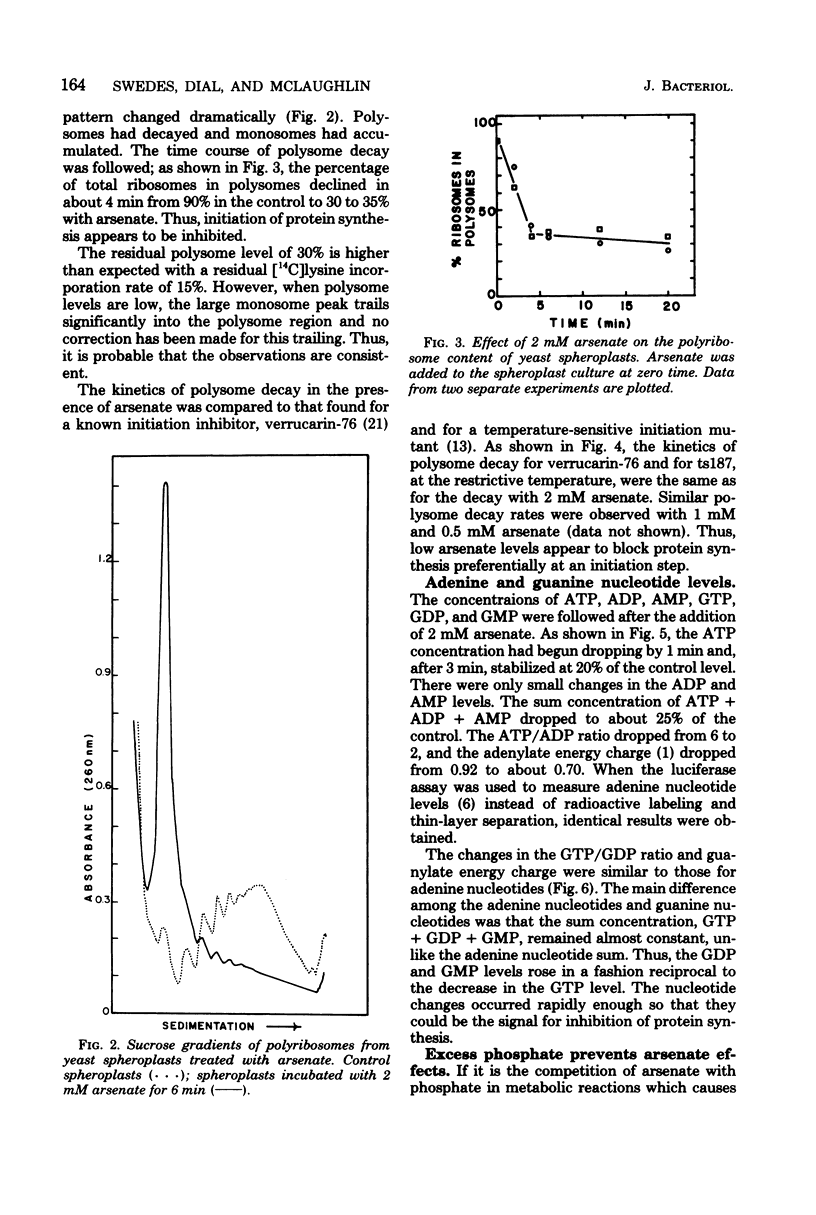
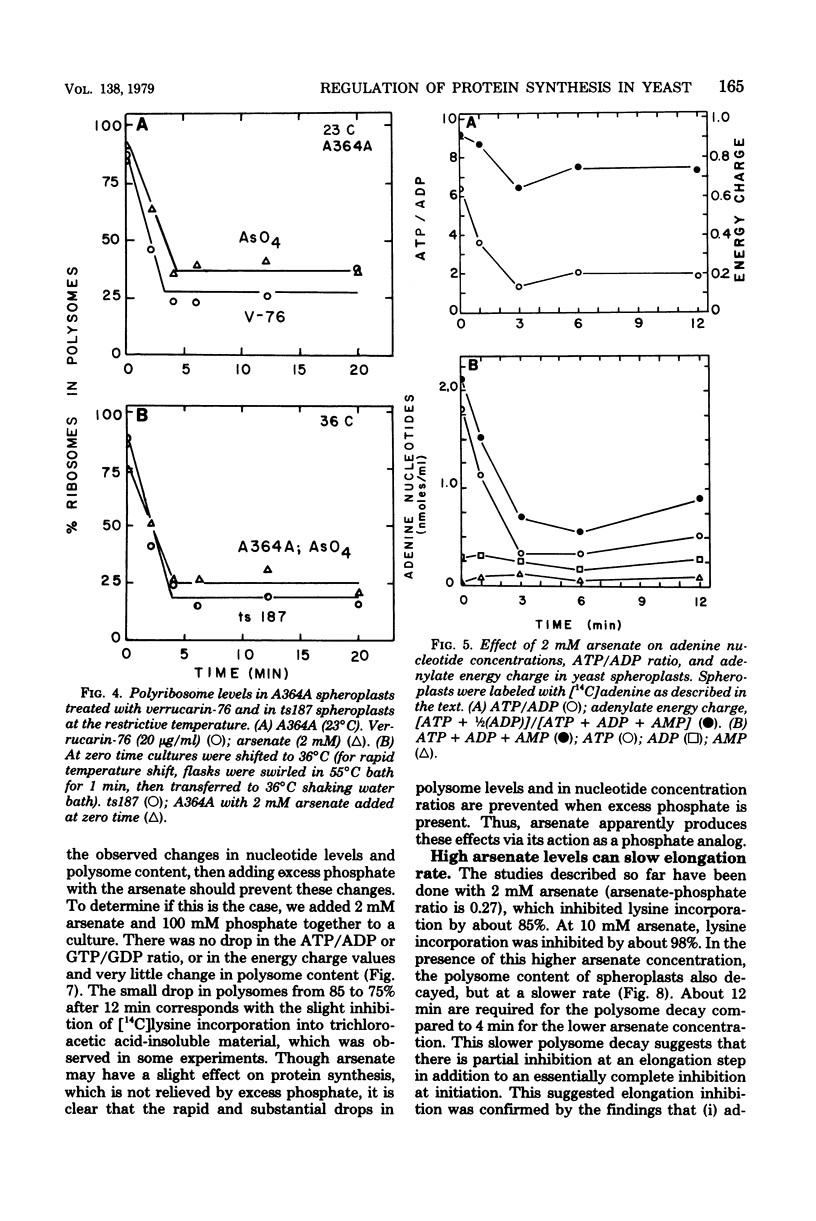
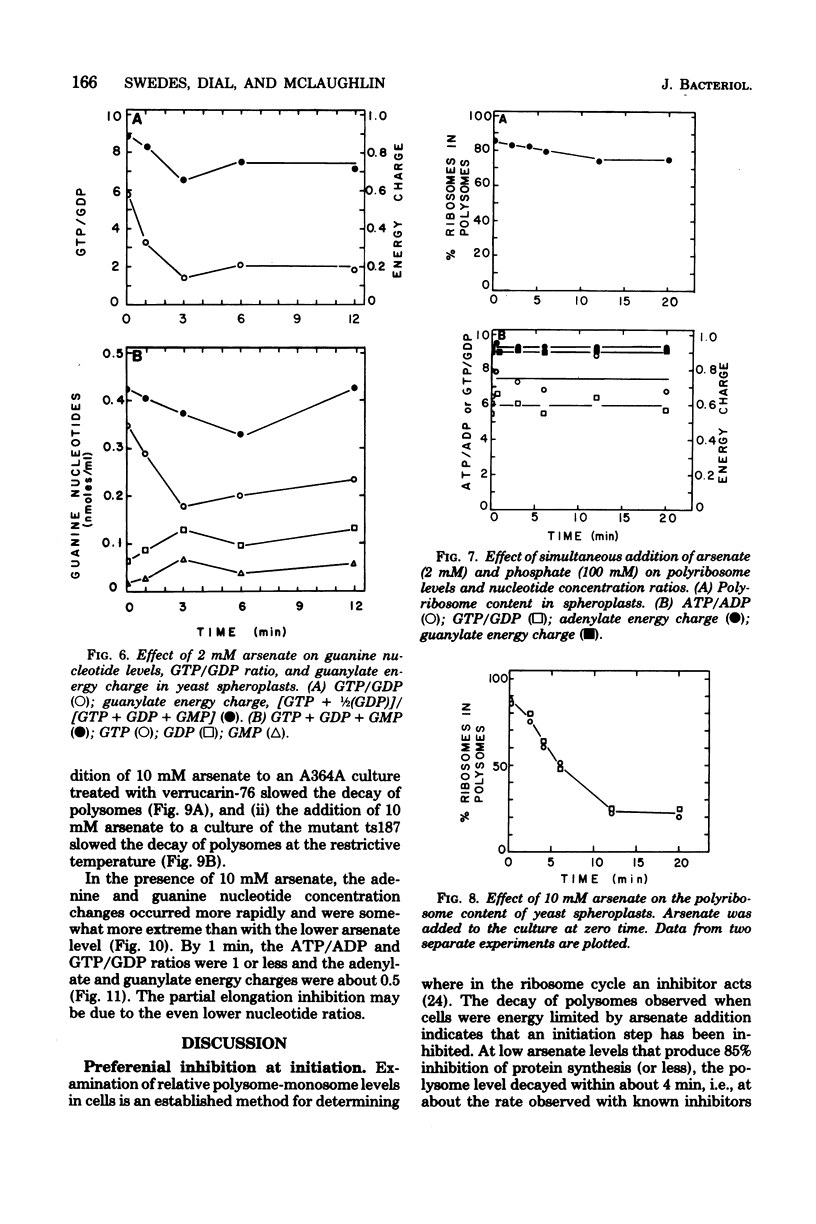
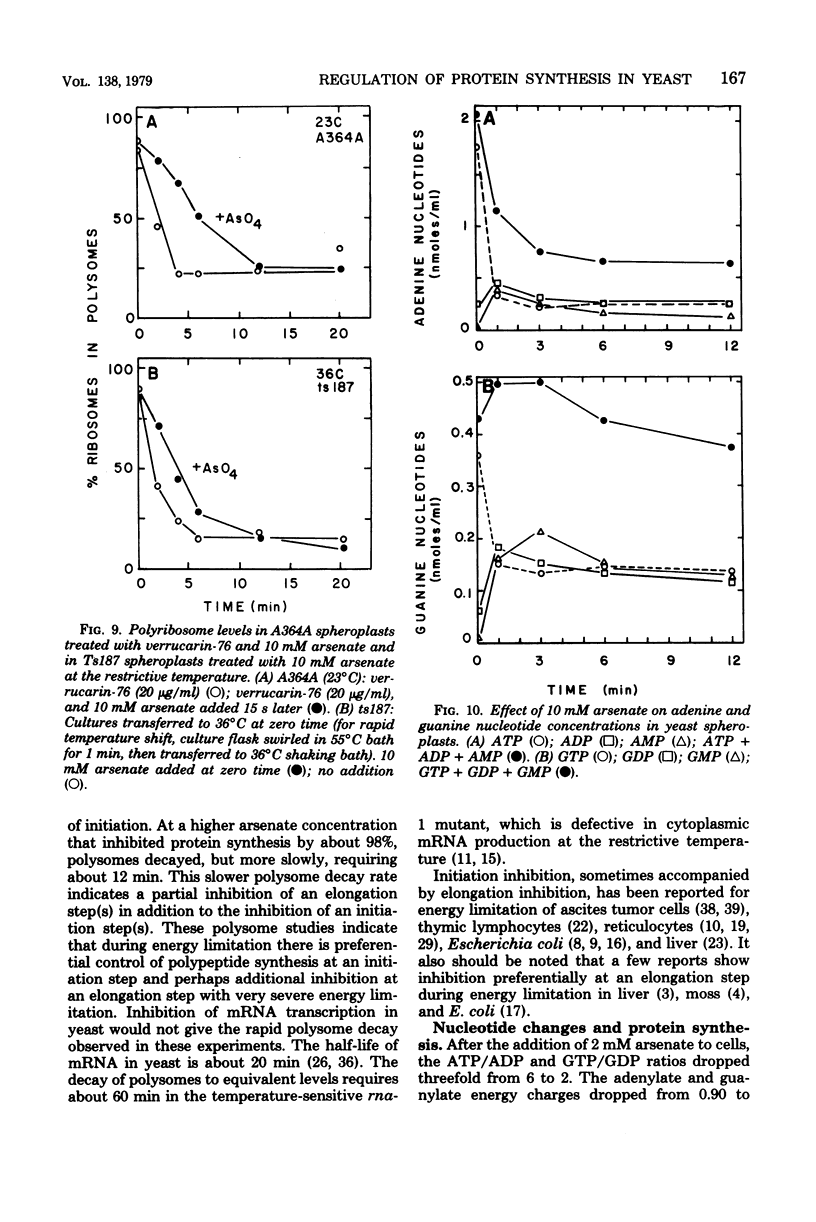
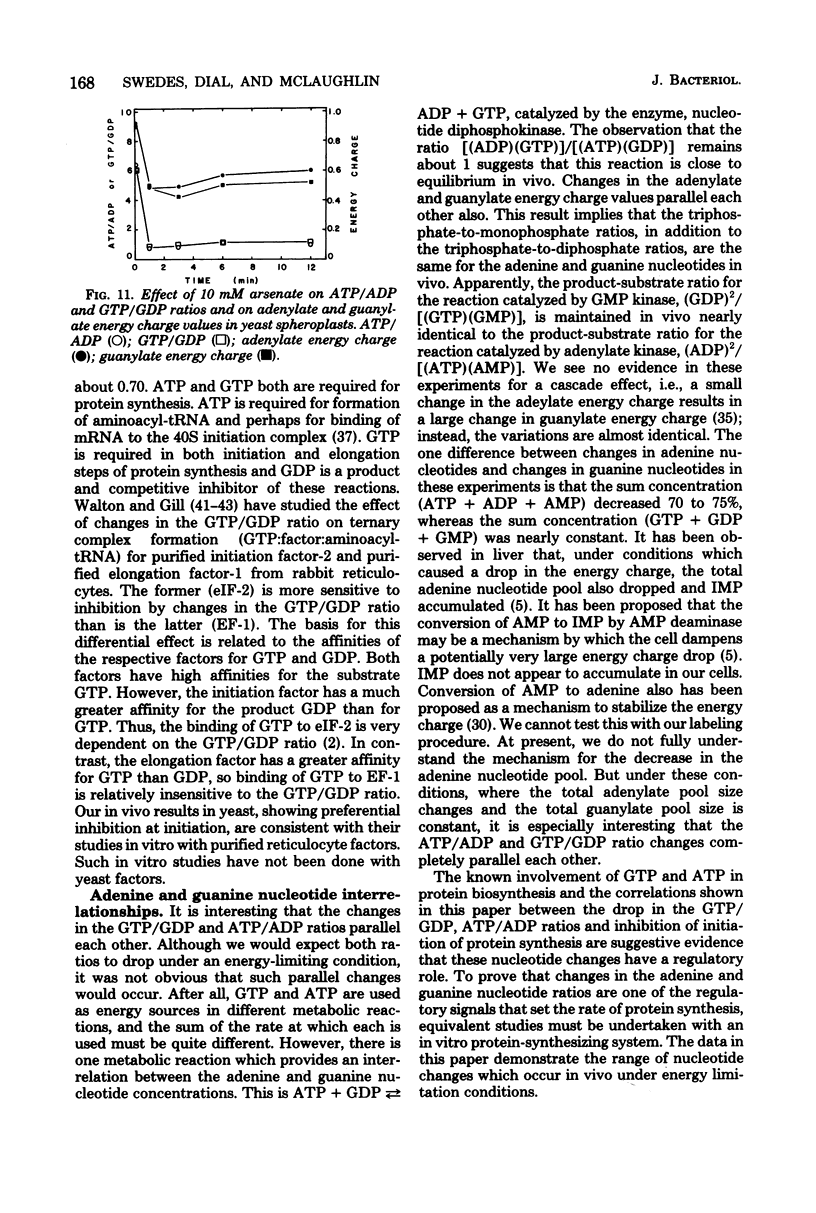
Arsenate, a competitive inhibitor with phosphate in phosphorylation reactions, has been used to lower adenine and guanine nucleotide levels in Saccharomyces cerevisiae to study nucleotide effects on protein synthesis. By measuring polysome levels, we have shown that initiation of protein synthesis is much more sensitive than elongation or termination to inhibition when the ATP/ADP, GTP/GDP ratios are low. When the arsenate-phosphate molar ratio was 0.27, protein synthesis was inhibited by about 85% and the kinetics of polysome decay was similar to that observed with the initiation inhibitor, verrucarin-76, or with the protein synthesis initiation mutant, ts187, at the restrictive temperature. With this level of arsenate, the adenylate energy charge dropped from 0.9 to 0.7 and the ATP/ADP and GTP/GDP ratios dropped from 6 to 2. The observed correlations between nucleotide ratio changes and inhibition of protein synthesis suggest that the former may be a control signal for the latter. The significance of these in vivo correlations will have to be tested with an in vitro protein synthesizing system. Higher arsenate levels resulted in even lower ATP/ADP, GTP/GDP ratios and in a slower decay of polysomes, implying that, eventually, elongation (in addition to initiation) was being inhibited.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E., Roach P. J., Schwedes J. S. Metabolite concentrations and concentration ratios in metabolic regulation. Adv Enzyme Regul. 1975;13:393–411. doi: 10.1016/0065-2571(75)90027-8. [DOI] [PubMed] [Google Scholar]
- Ayuso-Parrilla M. S., Parrilla R. Control of hepatic protein synthesis. Differential effects of ATP levels on the initiation and elongation steps. Eur J Biochem. 1975 Jul 15;55(3):593–599. doi: 10.1111/j.1432-1033.1975.tb02196.x. [DOI] [PubMed] [Google Scholar]
- Bewley J. D., Gwódź E. A. Plant Desiccation and Protein Synthesis: II. On the Relationship between Endogenous Adenosine Triphosphate Levels and Protein-synthesizing Capacity. Plant Physiol. 1975 Jun;55(6):1110–1114. doi: 10.1104/pp.55.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Stabilization of adenylate energy charge by the adenylate deaminase reaction. J Biol Chem. 1973 Dec 10;248(23):8309–8312. [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresden M. H., Hoagland M. B. Polyribosomes of Escherichia coli. Breakdown during glucose starvation. J Biol Chem. 1967 Mar 10;242(5):1065–1068. [PubMed] [Google Scholar]
- Friesen J. D. A study of the relationship between polyribosomes and messenger RNA in Escherichia coli. J Mol Biol. 1968 Mar 14;32(2):183–200. doi: 10.1016/0022-2836(68)90003-x. [DOI] [PubMed] [Google Scholar]
- Giloh H., Mager J. Inhibition of peptide chain initiation in lysates from ATP-depleted cells.I. Stages in the evolution of the lesion and its reversal by thiol compounds, cyclic AMP or purine derivatives and phosphorylated sugars. Biochim Biophys Acta. 1975 Dec 19;414(3):293–308. doi: 10.1016/0005-2787(75)90168-9. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Hutchison H. T., Holland T. M., McLaughlin C. S. The effect of cycloheximide upon polyribosome stability in two yeast mutants defective respectively in the initiation of polypeptide chains and in messenger RNA synthesis. Mol Gen Genet. 1970;106(4):347–361. doi: 10.1007/BF00324052. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. A mutant of yeast apparently defective in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1969 Feb;62(2):468–474. doi: 10.1073/pnas.62.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 1967 Nov;94(5):1697–1705. doi: 10.1128/jb.94.5.1697-1705.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H., McLaughlin C. S. Temperature-sensitive yeast mutant defective in ribonucleic acid production. J Bacteriol. 1969 Sep;99(3):807–814. doi: 10.1128/jb.99.3.807-814.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. A., Baldassare J. C. Association of messenger ribonucleic acid with 70S monosomes from down-shifted Escherichia coli. J Bacteriol. 1976 Jul;127(1):637–643. doi: 10.1128/jb.127.1.637-643.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen K., Molin S., Karlström O., Maaloe O. Control of protein synthesis in Escherichia coli: analysis of an energy source shift-down. J Bacteriol. 1977 Jul;131(1):18–29. doi: 10.1128/jb.131.1.18-29.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. W., Ray W. J., Jr Kinetics and thermodynamics of the formation of glucose arsenate. Reaction of glucose arsenate with phosphoglucomutase. Biochemistry. 1973 Sep 25;12(20):3932–3937. doi: 10.1021/bi00744a023. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Burka E. R., Conconi F. M., Perl W., Rifkind R. A. Polyribosome dissociation and formation in intact reticulocytes with conservation of messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1437–1443. doi: 10.1073/pnas.53.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Magee P. T., Hartwell L. H. Role of isoleucyl-transfer ribonucleic acid synthetase in ribonucleic acid synthesis and enzyme repression in yeast. J Bacteriol. 1969 Nov;100(2):579–584. doi: 10.1128/jb.100.2.579-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn S. L., Nordeen S. K., Young D. A. Rapid changes in initiation-limited rates of protein synthesis in rat thymic lymphocytes correlate with energy charge. Biochem Biophys Res Commun. 1977 Nov 7;79(1):53–60. doi: 10.1016/0006-291x(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Oler A., Farber E., Shull K. H. Resistance of liver polysomes to ATP deficiency in the male rat. Biochim Biophys Acta. 1969 Sep 17;190(1):161–169. doi: 10.1016/0005-2787(69)90165-8. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., McLaughlin C. S., Nierlich D. P. Half life of yeast messenger RNA. Nature. 1976 Mar 4;260(5546):70–72. doi: 10.1038/260070a0. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., McLaughlin C. S. Polysome metabolism in protein synthesis mutants of yeast. Mol Gen Genet. 1974 Mar 27;129(3):189–200. doi: 10.1007/BF00267912. [DOI] [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN A. Interactions of arsenate with the phosphate-transporting system of yeast. J Gen Physiol. 1963 May;46:1075–1085. doi: 10.1085/jgp.46.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak H. T., Quincey R. V. Small changes in energy charge affect protein synthesis in reticulocyte lysates. FEBS Lett. 1975 Oct 15;58(1):99–101. doi: 10.1016/0014-5793(75)80234-1. [DOI] [PubMed] [Google Scholar]
- SLOCUM D. H., VARNER J. E. Transfer of O18 in arsenolysis reactions. J Biol Chem. 1960 Feb;235:492–495. [PubMed] [Google Scholar]
- Schramm V. L., Leung H. Regulation of adenosine monophosphate levels as a function of adenosine triphosphate and inorganic phosphate. A proposed metabolic role for adenosine monophosphate nucleosidase from Azotobacter vinelandii. J Biol Chem. 1973 Dec 10;248(23):8313–8315. [PubMed] [Google Scholar]
- Sprague G. F., Jr Isolation and characterization of a Saccharomyces cerevisiae mutant deficient in pyruvate kinase activity. J Bacteriol. 1977 Apr;130(1):232–241. doi: 10.1128/jb.130.1.232-241.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford M. E., McLaughlin C. S. Trichodermin, a possible inhibitor of the termination process of protein synthesis. J Cell Physiol. 1973 Aug;82(1):121–128. doi: 10.1002/jcp.1040820114. [DOI] [PubMed] [Google Scholar]
- Thompson F. M., Atkinson D. E. Response of nucleoside diphosphate kinase to the adenylate energy charge. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1581–1585. doi: 10.1016/0006-291x(71)90201-4. [DOI] [PubMed] [Google Scholar]
- Tonnesen T., Friesen J. D. Inhibitors of ribonucleic acid synthesis in Saccharomyces cerevisiae: decay rate of messenger ribonucleic acid. J Bacteriol. 1973 Sep;115(3):889–896. doi: 10.1128/jb.115.3.889-896.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977 Nov;116(4):755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]
- Van Ventrooij W. J., Henshaw E. C., Hirsch C. A. Nutritional effects on the polyribosome distribution and rate of protein synthesis in Ehrlich ascites tumor cells in culture. J Biol Chem. 1970 Nov 25;245(22):5947–5953. [PubMed] [Google Scholar]
- Waldron C., Lacroute F. Effect of growth rate on the amounts of ribosomal and transfer ribonucleic acids in yeast. J Bacteriol. 1975 Jun;122(3):855–865. doi: 10.1128/jb.122.3.855-865.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Nucleotide regulation of a eukaryotic protein synthesis initiation complex;. Biochim Biophys Acta. 1975 May 1;390(2):231–245. doi: 10.1016/0005-2787(75)90344-5. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Preferential regulation of protein synthesis initiation complex formation by purine nucleotides. Biochim Biophys Acta. 1976 Sep 20;447(1):11–19. doi: 10.1016/0005-2787(76)90090-3. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Regulation of ternary (Met-tRNAf - GTP - eukaryotic initiation factor 2) protein synthesis initiation complex formation by the adenylate energy charge. Biochim Biophys Acta. 1976 Jan 19;418(2):195–203. doi: 10.1016/0005-2787(76)90069-1. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Hansen B. S., Vaughan M. H., Jr, McLaughlin C. S. Mechanism of action of the mycotoxin trichodermin, a 12,13-epoxytrichothecene. Proc Natl Acad Sci U S A. 1974 Mar;71(3):713–717. doi: 10.1073/pnas.71.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel K. E., Mor J. R., Fiechter A. Rapid sampling of yeast cells and automated assays of adenylate, citrate, pyruvate and glucose-6-phosphate pools. Anal Biochem. 1974 Mar;58(1):208–216. doi: 10.1016/0003-2697(74)90459-x. [DOI] [PubMed] [Google Scholar]
- ter Welle H. F., Slater E. C. Uncoupling of respiratory-chain phosphorylation by arsenate. Biochim Biophys Acta. 1967 Jul 5;143(1):1–17. doi: 10.1016/0005-2728(67)90104-1. [DOI] [PubMed] [Google Scholar]


