Abstract
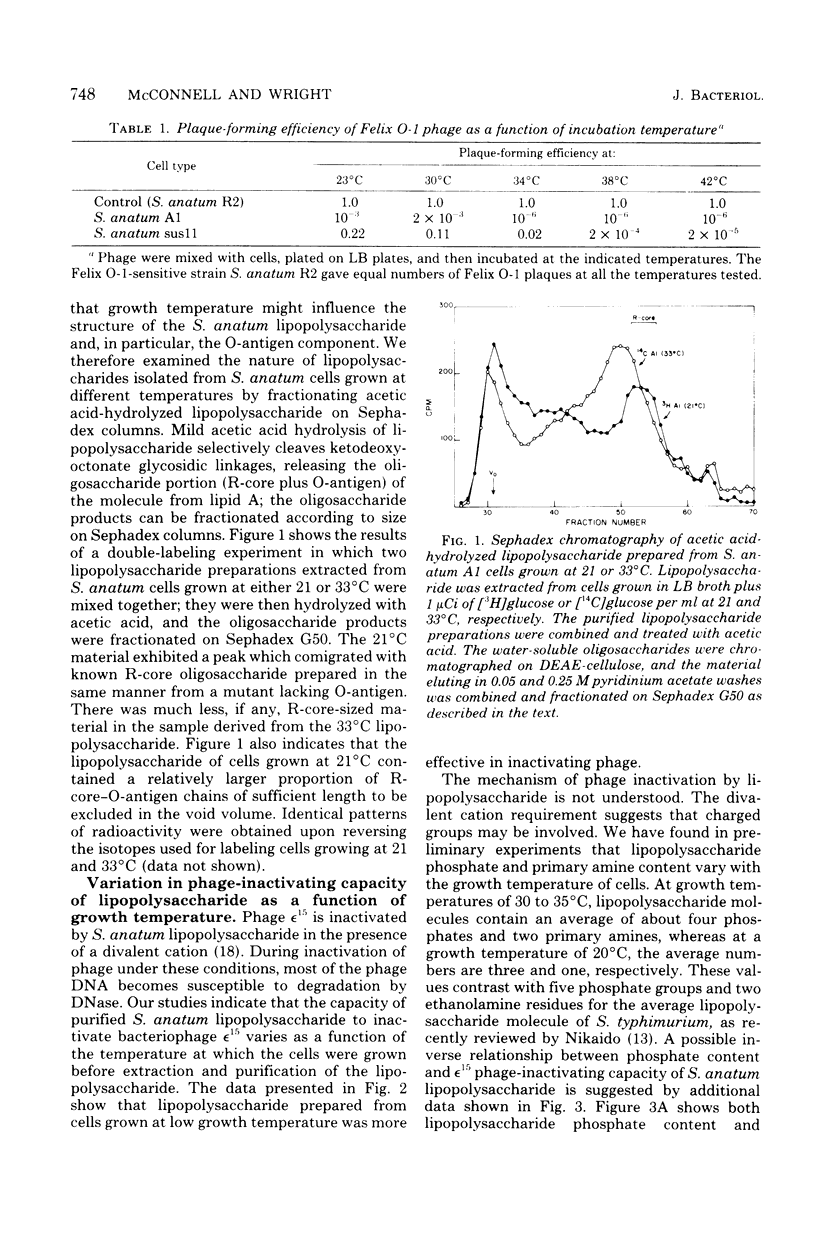
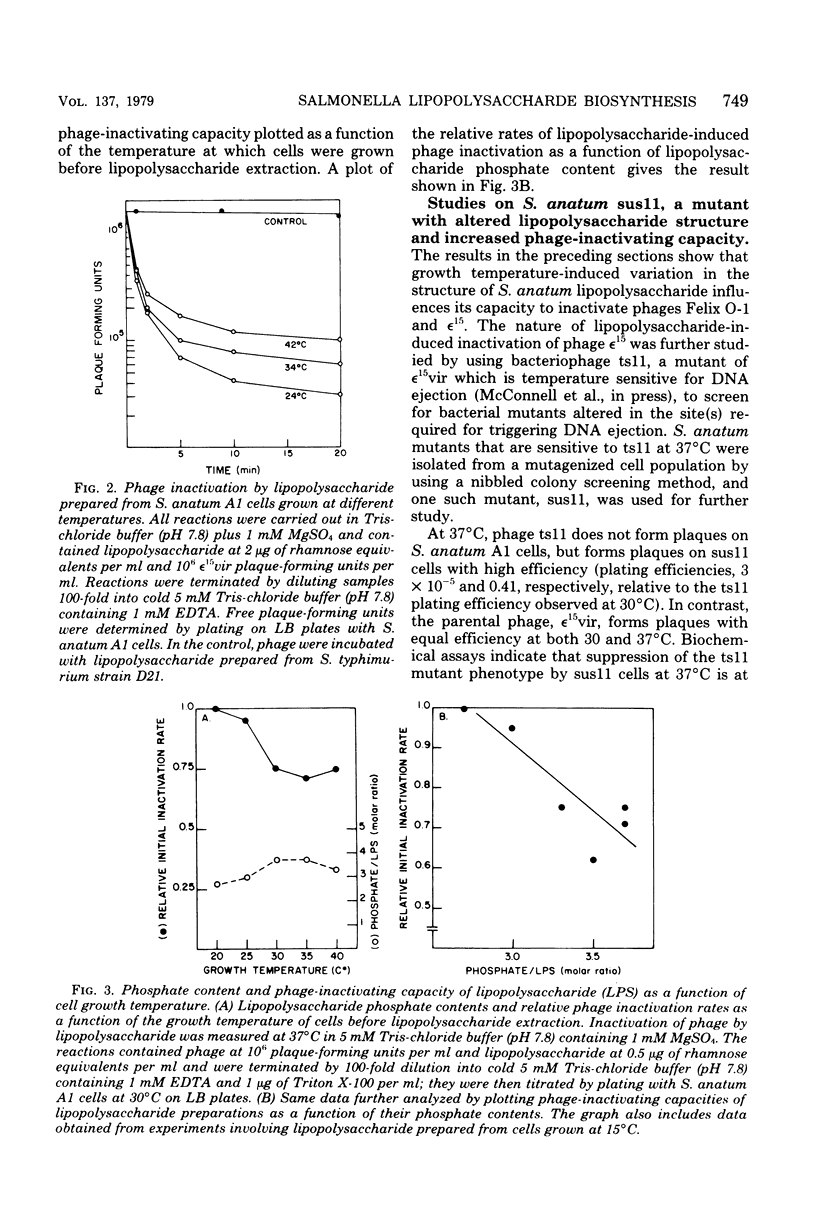
Growth temperature affects both the structure and the phage-inactivating capacity of Salmonella anatum A1 lipopolysaccharide. Whereas S. anatum cells normally synthesize smooth lipopolysaccharide when grown at physiological temperature (37 degrees C), a partial smooth-rough transition occurs when cells are grown at low temperature (20 to 25 degrees C). The synthesis at low growth temperature of lipopolysaccharide molecules lacking O-antigen was detected both by increased sensitivity of cells to the rough-specific bacteriophage Felix O-1 and by fractionation of oligosaccharides derived from lipopolysaccharide by mild acid hydrolysis. Growth temperature-induced changes in the structure of S. anatum A1 lipopolysaccharide also affected its ability to inactivate epsilon15, a bacteriophage that binds initially to the O-antigen portion of the molecule. Purified lipopolysaccharide prepared from cells grown at low growth temperature exhibited a higher in vitro phage-inactivating capacity than did lipopolysaccharide prepared from cells grown at physiological temperature (37 degrees C).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMANN I., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. II. SEROLOGICAL AND CHEMICAL INVESTIGATIONS. Nature. 1964 Mar 28;201:1299–1301. doi: 10.1038/2011299a0. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Properties of the O-specific hapten formed in vivo by mutant strains of Salmonella typhimurium. Biochemistry. 1968 Dec;7(12):4396–4408. doi: 10.1021/bi00852a036. [DOI] [PubMed] [Google Scholar]
- Khesin R. B., Mindlin S. Z., Gorlenko Z. M., Ilyina T. S. Temperature sensitive mutations affecting RNA synthesis in Escherichia coli. Mol Gen Genet. 1968;103(2):194–208. doi: 10.1007/BF00427146. [DOI] [PubMed] [Google Scholar]
- Koeltzow D. E., Conrad H. E. Structural heterogeneity in the lipopolysaccharide of Aerobacter aerogenes NCTC 243. Biochemistry. 1971 Jan 19;10(2):214–224. doi: 10.1021/bi00778a004. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Influence of O side chains on the attachment of the Felix O-1 bacteriophage to Salmonella bacteria. J Bacteriol. 1969 Aug;99(2):513–519. doi: 10.1128/jb.99.2.513-519.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Structure of cell wall lipopolysaccharide from Salmonella typhimurium. Further studies on the linkage between O side chains and R core. Eur J Biochem. 1970 Jul;15(1):57–62. doi: 10.1111/j.1432-1033.1970.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- ROBBINS P. W., UCHIDA T. Studies on the chemical basis of the phage conversion of O-antigens in the E-group Salmonellae. Biochemistry. 1962 Mar;1:323–335. doi: 10.1021/bi00908a020. [DOI] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Uetake H. In vitro interaction between phage and receptor lipopolysaccharide: a novel glycosidase associated with Salmonella phage epsilon15. Virology. 1973 Mar;52(1):148–159. [PubMed] [Google Scholar]
- Weiner I. M., Higuchi T., Rothfield L., Saltmarsh-Andrew M., Osborn M. J., Horecker B. L. Biosynthesis of bacterial lipopolysaccharide. V. Lipid-linked intermediates in the biosynthesis of the O-antigen groups of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1965 Jul;54(1):228–235. doi: 10.1073/pnas.54.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Barzilai N. Isolation and haracterization nonconverting mutants of bacteriophage epsilon 34. J Bacteriol. 1971 Mar;105(3):937–939. doi: 10.1128/jb.105.3.937-939.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Robbins P. W. Evidence for an intermediate stage in the biosynthesis of the Salmonella O-antigen. Proc Natl Acad Sci U S A. 1965 Jul;54(1):235–241. doi: 10.1073/pnas.54.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Kanegasaki S. Molecular aspects of lipopolysaccharides. Physiol Rev. 1971 Oct;51(4):748–784. doi: 10.1152/physrev.1971.51.4.748. [DOI] [PubMed] [Google Scholar]
- Wright A. Mechanism of conversion of the salmonella O antigen by bacteriophageepsilon 34. J Bacteriol. 1971 Mar;105(3):927–936. doi: 10.1128/jb.105.3.927-936.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]


