Abstract
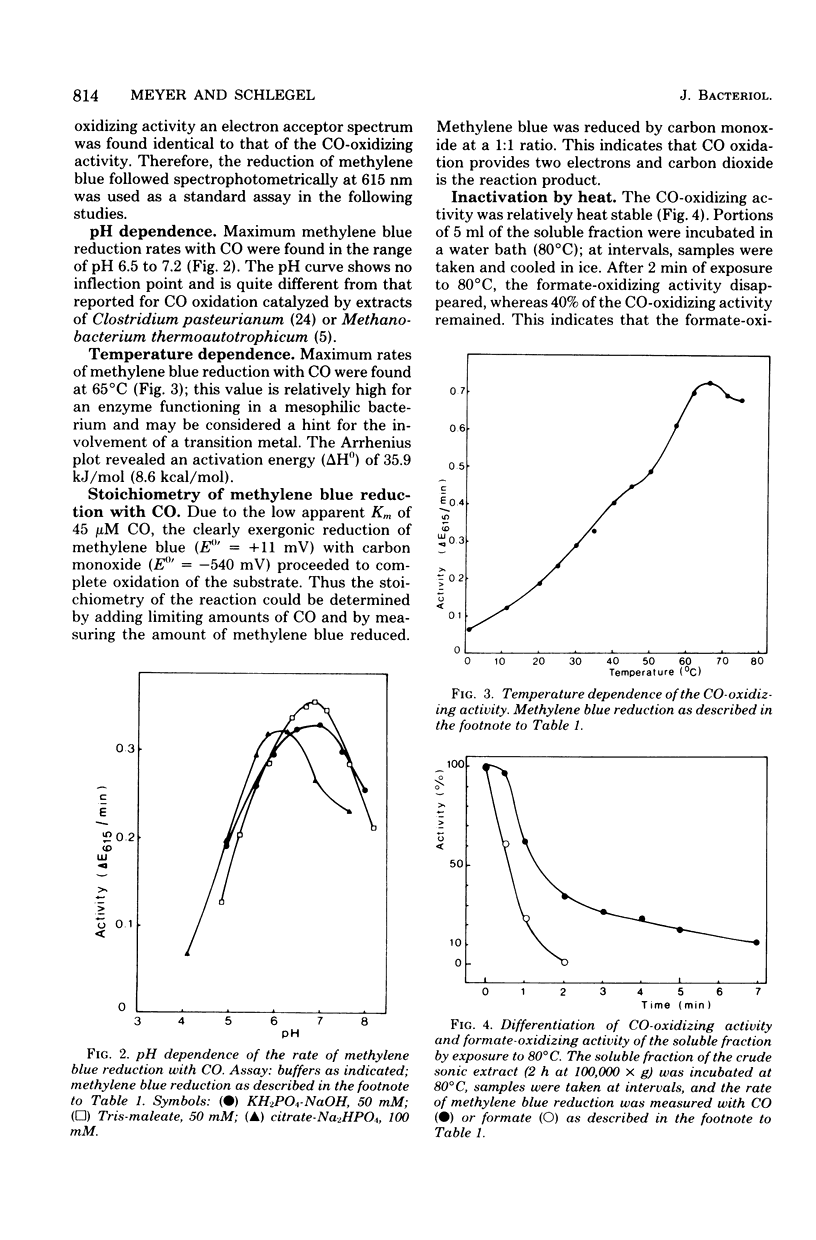
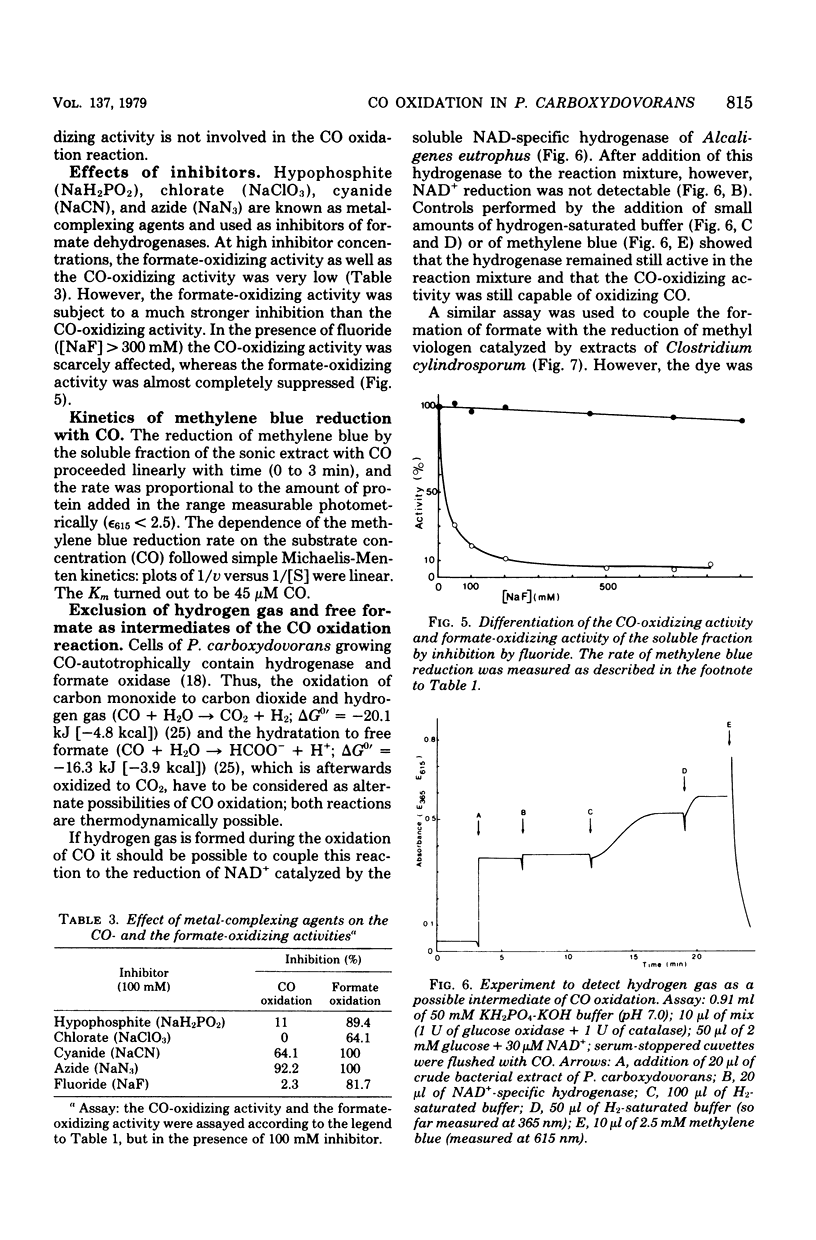
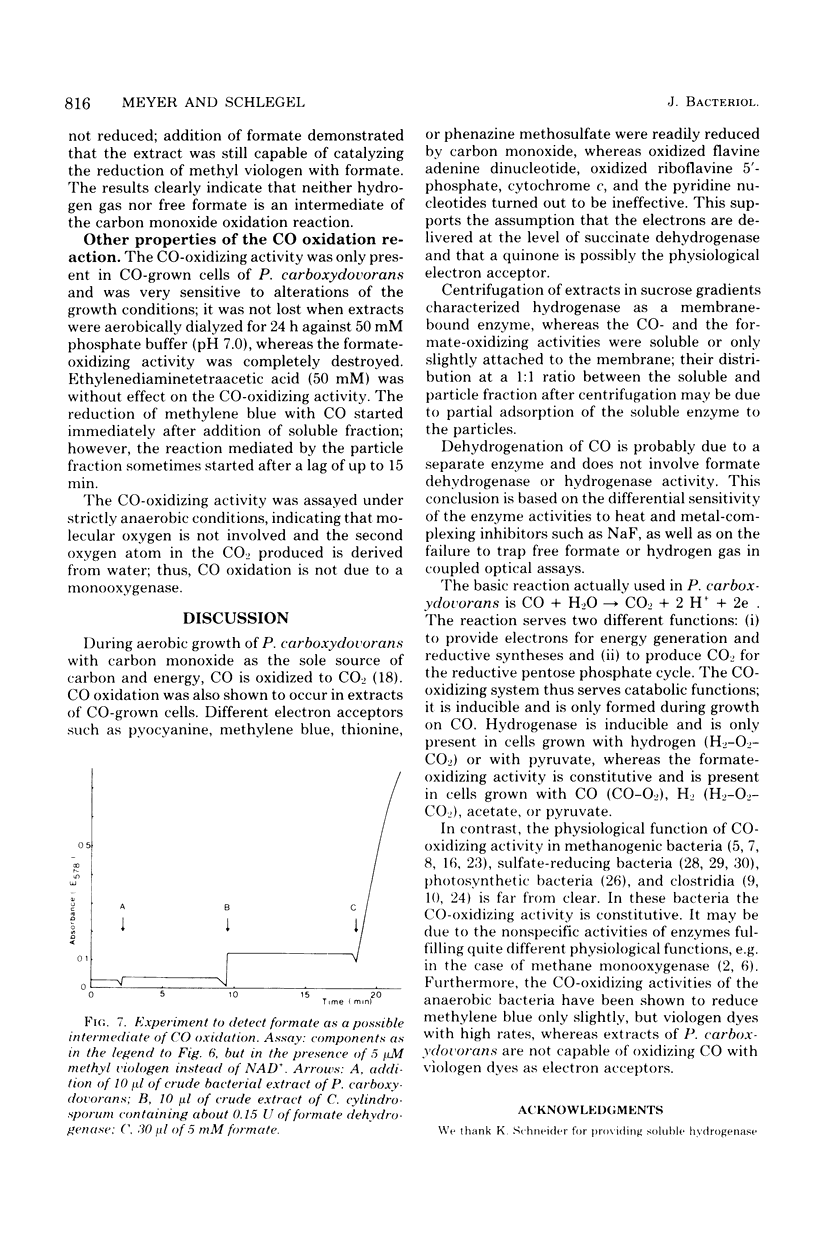
Extracts of aerobically, CO-autotrophically grown cells of Pseudomonas carboxydovorans were shown to catalyze the oxidation of CO to CO2 in the presence of methylene blue, pyocyanine, thionine, phenazine methosulfate, or toluylene blue under strictly anaerobic conditions. Viologen dyes and NAD(P)+ were ineffective as electron acceptors. The same extracts catalyzed the oxidation of formate and of hydrogen gas; the spectrum of electron acceptors was identical for the three substrates, CO, formate, and H2. The CO- and the formate-oxidizing activities were found to be soluble enzymes, whereas hydrogenase was membrane bound exclusively. The rates of oxidation of CO, formate, and H2 were measured spectrophotometrically following the reduction of methylene blue. The rate of carbon monoxide oxidation followed simple Michaelis-Menten kinetics; the apparent Km for CO was 45 μM. The reaction rate was maximal at pH 7.0, and the temperature dependence followed the Arrhenius equation with an activation energy (ΔH0) of 35.9 kJ/mol (8.6 kcal/mol). Neither free formate nor hydrogen gas is an intermediate of the CO oxidation reaction. This conclusion is based on the differential sensitivity of the activities of formate dehydrogenase, hydrogenase, and CO dehydrogenase to heat, hypophosphite, chlorate, cyanide, azide, and fluoride as well as on the failure to trap free formate or hydrogen gas in coupled optical assays. These results support the following equation for CO oxidation in P. carboxydovorans: CO + H2O → CO2 + 2 H+ + 2e− The CO-oxidizing activity of P. carboxydovorans differed from that of Clostridium pasteurianum by not reducing viologen dyes and by a pH optimum curve that did not show an inflection point.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggag M., Schlegel H. G. Studies on a gram-positive hydrogen bacterium, Nocardia opaca 1 b. III. Purification, stability and some properties of the soluble hydrogen dehydrogenase. Arch Microbiol. 1974;100(1):25–39. doi: 10.1007/BF00446303. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- Fuchs G., Schnitker U., Thauer R. K. Carbon monoxide oxidation by growing cultures of Clostridium pasteurianum. Eur J Biochem. 1974 Nov 1;49(1):111–115. doi: 10.1111/j.1432-1033.1974.tb03816.x. [DOI] [PubMed] [Google Scholar]
- Hubley J. H., Mitton J. R., Wilkinson J. F. The oxidation of carbon monoxide by methane-oxidizing bacteria. Arch Mikrobiol. 1974 Feb 13;95(4):365–368. doi: 10.1007/BF02451778. [DOI] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Reisolation of the carbon monoxide utilizing hydrogen bacterium Pseudomonas carboxydovorans (Kistner) comb. nov. Arch Microbiol. 1978 Jul;118(1):35–43. doi: 10.1007/BF00406071. [DOI] [PubMed] [Google Scholar]
- Nozhevnikova A. N., Zavarzin G. A. K taksonomii CO-okisliaiushchikh gramotritsatel'nykh bakterii. Izv Akad Nauk SSSR Biol. 1974 May-Jun;(3):436–440. [PubMed] [Google Scholar]
- Sanzhieva E. U., Zavarzin G. A. Bakteriia, okisliaiushchaia okis' ugleroda. Dokl Akad Nauk SSSR. 1971 Feb 1;196(4):956–958. [PubMed] [Google Scholar]
- Savel'eva N. D., Nozhevnikova A. N. Avtotrofnyi rost Seliberia carboxydohydrogena pri okislenii vodoroda i okisi ugleroda. Mikrobiologiia. 1972 Sep-Oct;41(5):813–817. [PubMed] [Google Scholar]
- Stephenson M., Stickland L. H. Hydrogenase: The bacterial formation of methane by the reduction of one-carbon compounds by molecular hydrogen. Biochem J. 1933;27(5):1517–1527. doi: 10.1042/bj0271517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Fuchs G., Käufer B., Schnitker U. Carbon-monoxide oxidation in cell-free extracts of Clostridium pasteurianum. Eur J Biochem. 1974 Jun 15;45(2):343–349. doi: 10.1111/j.1432-1033.1974.tb03559.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGI T. Enzymic oxidation of carbon monoxide. Biochim Biophys Acta. 1958 Oct;30(1):194–195. doi: 10.1016/0006-3002(58)90263-4. [DOI] [PubMed] [Google Scholar]
- YAGI T., TAMIYA N. Enzymic oxidation of carbon monoxide. III. Reversibility. Biochim Biophys Acta. 1962 Dec 17;65:508–509. doi: 10.1016/0006-3002(62)90454-7. [DOI] [PubMed] [Google Scholar]


