Abstract
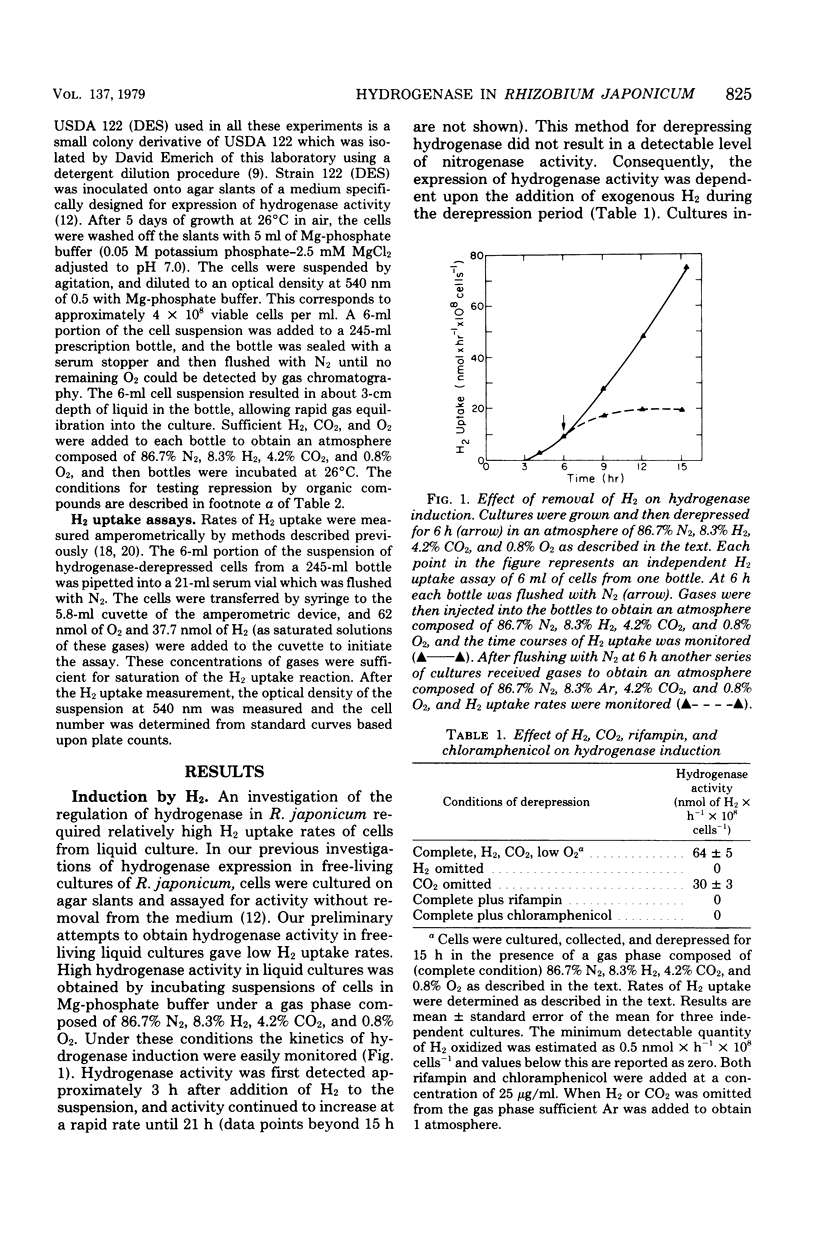
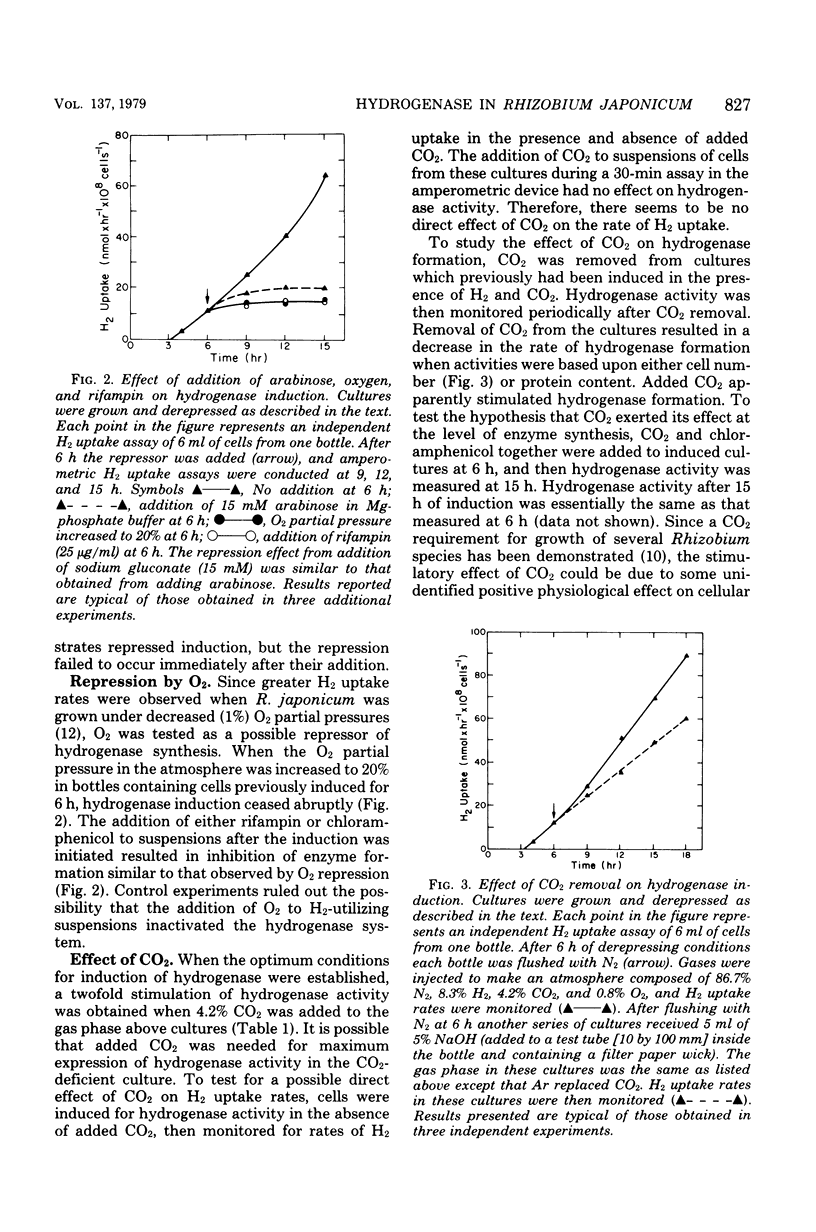
Factors that regulate the expression of an H2 uptake system in free-living cultures of Rhizobium japonicum have been investigated. Rapid rates of H2 uptake by R. japonicum were obtained by incubation of cell suspensions in a Mg-phosphate buffer under a gas phase of 86.7% N2, 8.3% H2, 4.2% CO2, and 0.8% O2. Cultures incubated under conditions comparable with those above, with the exception that Ar replaced H2, showed no hydrogenase activity. When H2 was removed after initiation of hydrogenase derepression, further increase in hydrogenase activity ceased. Nitrogenase activity was not essential for expression of hydrogenase activity. All usable carbon substrates tested repressed hydrogenase formation, but none of them inhibited hydrogenase activity. No effect on hydrogenase formation was observed from the addition of KNO3 or NH4Cl at 10 mM. Oxygen repressed hydrogenase formation, but did not inhibit activity of the enzyme in whole cells. The addition of rifampin or chloramphenicol to derepressed cultures resulted in inhibition of enzyme formation similar to that observed by O2 repression. The removal of CO2 during derepression caused a decrease in the rate of hydrogenase formation. No direct effect of CO2 on hydrogenase activity was observed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bongers L. Energy generation and utilization in hydrogen bacteria. J Bacteriol. 1970 Oct;104(1):145–151. doi: 10.1128/jb.104.1.145-151.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. R., Jennings N. T., Hanus J., Evans H. J. Hydrogen evolution and uptake by nodules of soybeans inoculated with different strains of Rhizobium japonicum. Can J Microbiol. 1978 Mar;24(3):307–311. doi: 10.1139/m78-051. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Ruiz-Argüeso T., Jennings N., Hanus J. Energy coupling efficiency of symbiotic nitrogen fixation. Basic Life Sci. 1977;9:333–354. doi: 10.1007/978-1-4684-0880-5_21. [DOI] [PubMed] [Google Scholar]
- Kuykendall L. D., Elkan G. H. Rhizobium japonicum derivatives differing in nitrogen-fixing efficiency and carbohydrate utilization. Appl Environ Microbiol. 1976 Oct;32(4):511–519. doi: 10.1128/aem.32.4.511-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae R. E., Hanus J., Evans H. J. Properties of the hydrogenase system in Rhizobium japonicum bacteroids. Biochem Biophys Res Commun. 1978 Jan 30;80(2):384–390. doi: 10.1016/0006-291x(78)90688-5. [DOI] [PubMed] [Google Scholar]
- O'gara F., Shanmugam K. T. Mutant strains of clover rhizobium (Rhizobium trifolii) that form nodules on soybean (Glycine max). Proc Natl Acad Sci U S A. 1978 May;75(5):2343–2347. doi: 10.1073/pnas.75.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Ortiz J. M., Burris R. H. Interactions among substrates and inhibitors of nitrogenase. J Bacteriol. 1975 Aug;123(2):537–545. doi: 10.1128/jb.123.2.537-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. Mutants of Alcaligenes eutrophus defective in autotrophic metabolism. Arch Microbiol. 1978 May 30;117(2):123–129. doi: 10.1007/BF00402299. [DOI] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Jennings N. T., Evans H. J. Hydrogen Reactions of Nodulated Leguminous Plants: II. Effects on Dry Matter Accumulation and Nitrogen Fixation. Plant Physiol. 1978 Mar;61(3):398–401. doi: 10.1104/pp.61.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Shah V. K., Brill W. J. Regulation of nitrogenase synthesis by oxygen in Klebsiella pneumoniae. J Bacteriol. 1974 Jul;119(1):266–269. doi: 10.1128/jb.119.1.266-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Healey F. P., Myers J. Amperometric measurement of hydrogen evolution in chlamydomonas. Plant Physiol. 1971 Jul;48(1):108–110. doi: 10.1104/pp.48.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


