Abstract
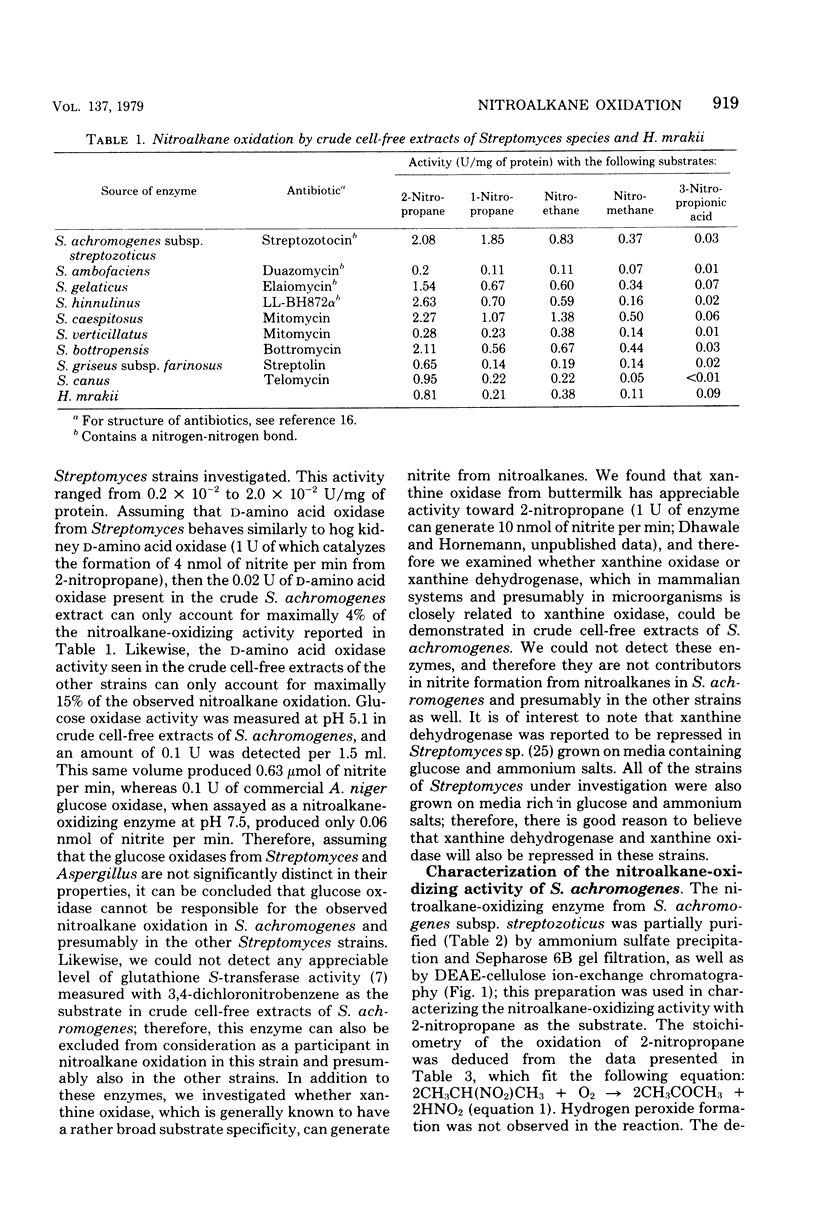
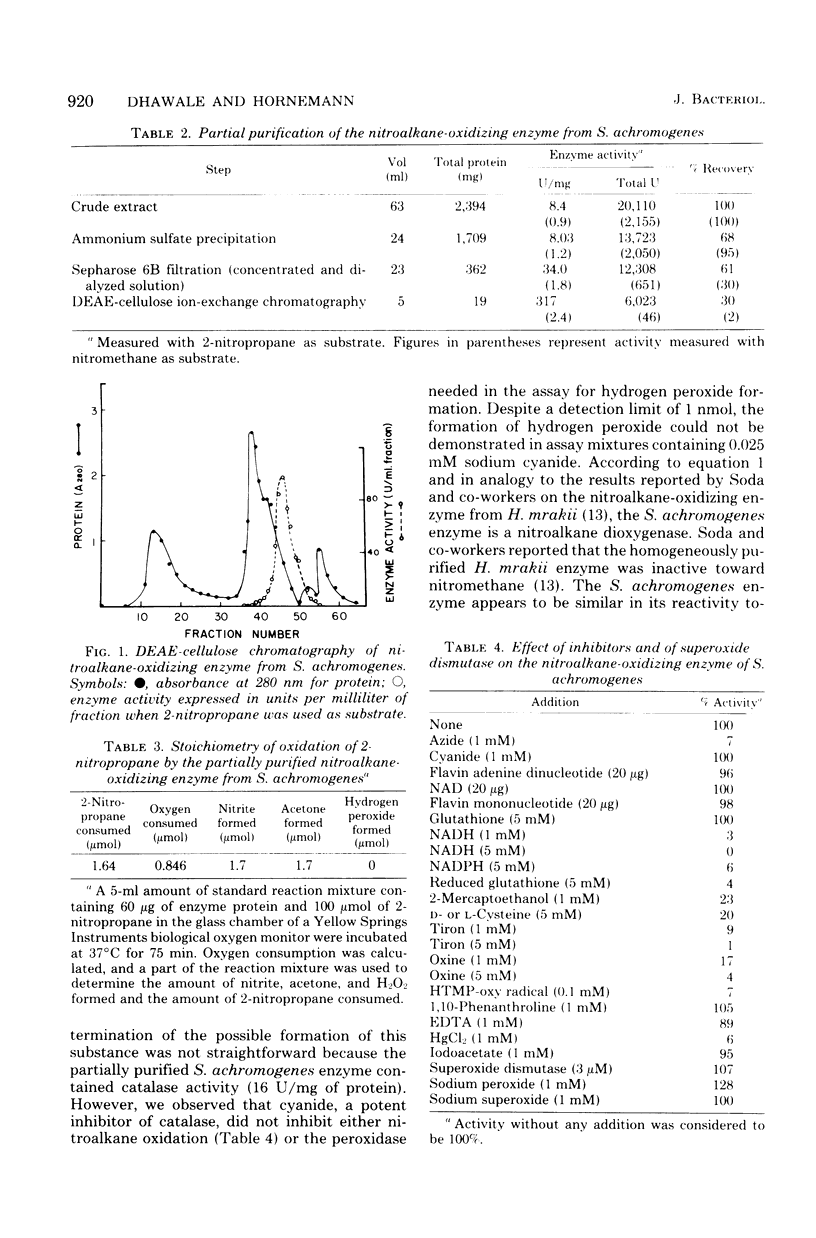
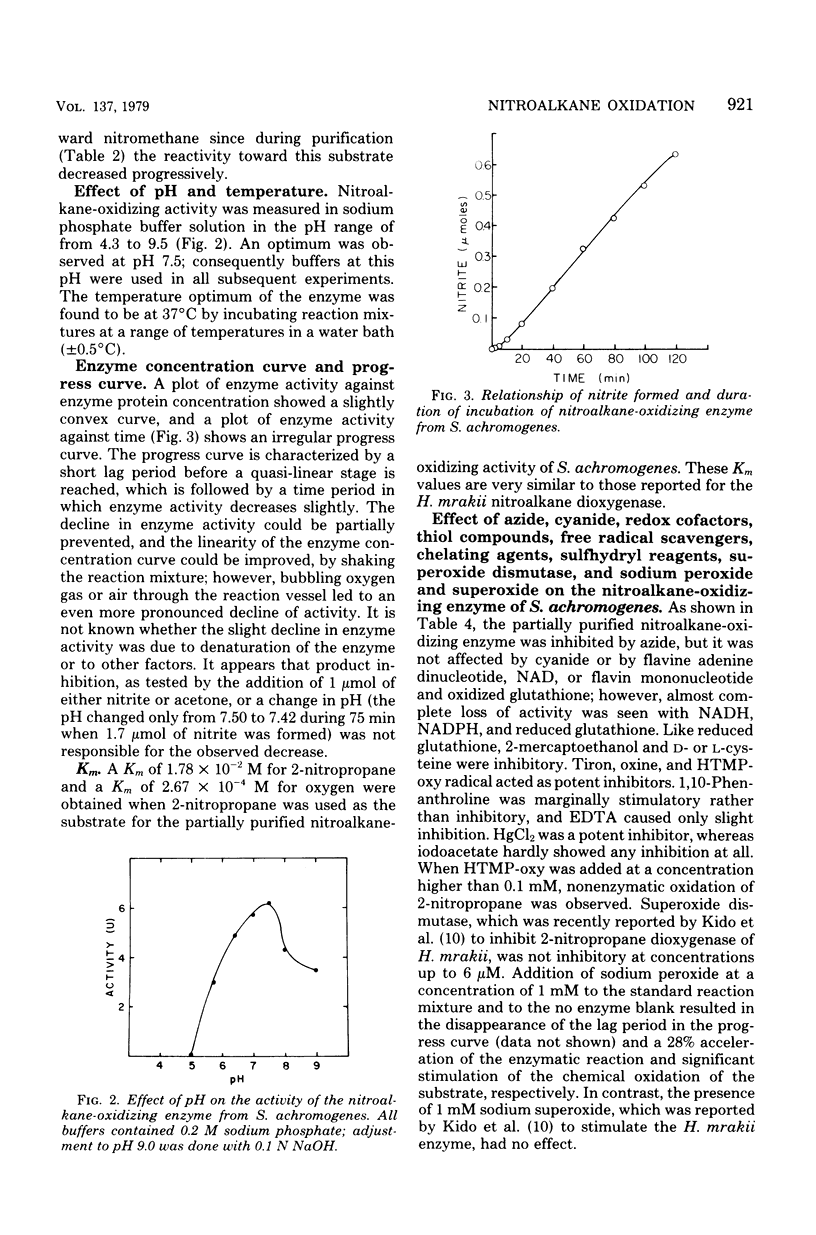
Crude cell-free extracts of nine strains of Streptomyces tested for nitroalkane-oxidizing activity showed production of nitrous acid from 2-nitropropane, 1-nitropropane, nitroethane, nitromethane, and 3-nitropropionic acid. These substrates were utilized in most strains but to a decreasing extent in the order given, and different strains varied in their relative efficiency of oxidation. p-Nitrobenzoic acid, p-aminobenzoic acid, enteromycin, and ω-nitro-l-arginine were not attacked. d-Amino acid oxidase, glucose oxidase, glutathione S-transferase, and xanthine oxidase, enzymes potentially responsible for the observed oxidations in crude cellfree extracts, were present at concentrations too low to play any significant role. A nitroalkane-oxidizing enzyme from streptozotocin-producing Streptomyces achromogenes subsp. streptozoticus was partially purified and characterized. It catalyzes the oxidative denitrification of 2-nitropropane as follows: 2CH3CH(NO2)CH3 + O2 → 2CH3COCH3 + 2HNO2. At the optimum pH of 7.5 of the enzyme, 2-nitropropane was as good a substrate as its sodium salt; t-nitrobutane was not a substrate. Whereas Tiron, oxine, and nitroxyl radical acted as potent inhibitors of this enzyme, superoxide dismutase was essentially without effect. Sodium peroxide abolished a lag phase in the progress curve of the enzyme and afforded stimulation, whereas sodium superoxide did not affect the reaction. Reducing agents, such as glutathione, reduced nicotinamide adenine dinucleotide, and nicotinamide adenine dinucleotide phosphate, reduced form, as well as thiol compounds, were strongly inhibitory, but cyanide had no effect. The S. achromogenes enzyme at the present stage of purification is similar in many respects to the enzyme 2-nitropropane dioxygenase from Hansenula mrakii. The possible involvement of the nitroalkane-oxidizing enzyme in the biosynthesis of antibiotics that contain a nitrogen-nitrogen bond is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANZAI K., SUZUKI S. A new antibiotic bovinocidin, identified as beta-nitropropionic acid. J Antibiot (Tokyo) 1960 Mar;13:133–136. [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- BUSH M. T., TOUSTER O., BROCKMAN J. E. The production of beta-nitropropionic acid by a strain of Aspergillus flavus. J Biol Chem. 1951 Feb;188(2):685–693. [PubMed] [Google Scholar]
- Chan T. W., Bruice T. C. One and two electron transfer reactions of glucose oxidase. J Am Chem Soc. 1977 Mar 30;99(7):2387–2389. doi: 10.1021/ja00449a084. [DOI] [PubMed] [Google Scholar]
- Collins-Thompson D. L., Sen N. P., Aris B., Schwinghamer L. Non-enzymic in vitro formation of nitrosamines by bacteria isolated from meat products. Can J Microbiol. 1972 Dec;18(12):1968–1971. doi: 10.1139/m72-306. [DOI] [PubMed] [Google Scholar]
- Gruner B. J., DeAngelo A. B., Shaw P. D. The isolation and some properties of an enzyme system which catalyzes the degradation of -nitropropionic acid. Arch Biochem Biophys. 1972 Jan;148(1):107–114. doi: 10.1016/0003-9861(72)90121-x. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- KIRSCH E. J., KORSHALLA J. D. INFLUENCE OF BIOLOGICAL METHYLATION ON THE BIOSYNTHESIS OF MITOMYCIN A. J Bacteriol. 1964 Feb;87:247–255. doi: 10.1128/jb.87.2.247-255.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido T., Hashizume K., Soda K. Purification and properties of nitroalkane oxidase from Fusarium oxysporum. J Bacteriol. 1978 Jan;133(1):53–58. doi: 10.1128/jb.133.1.53-58.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido T., Soda K. Properties of 2-nitropropane dioxygenase of Hansenula mrakii. Formation and participation of superoxide. J Biol Chem. 1978 Jan 10;253(1):226–232. [PubMed] [Google Scholar]
- Kido T., Soda K., Suzuki T., Asada K. A new oxygenase, 2-nitropropane dioxygenase of Hansenula mrakii. Enzymologic and spectrophotometric properties. J Biol Chem. 1976 Nov 25;251(22):6994–7000. [PubMed] [Google Scholar]
- Kido T., Yamamoto T., Soda K. Microbial assimilation of alkyl nitro compounds and formation of nitrite. Arch Microbiol. 1975 Dec 31;106(3):165–169. doi: 10.1007/BF00446519. [DOI] [PubMed] [Google Scholar]
- Kido T., Yamamoto T., Soda K. Purification and properties of nitroalkane-oxidizing enzyme from Hansenula mrakii. J Bacteriol. 1976 Jun;126(3):1261–1265. doi: 10.1128/jb.126.3.1261-1265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLE H. N. Oxidation of nitroethane by extracts from Neurospora. J Biol Chem. 1951 Nov;193(1):347–358. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRue T. A. Naturally occurring compounds Containing a nitrogen-nitrogen bond. Lloydia. 1977 Jul-Aug;40(4):307–321. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mills A. L., Alexander M. N-Nitrosamine formation by cultures of several microorganisms. Appl Environ Microbiol. 1976 Jun;31(6):892–895. doi: 10.1128/aem.31.6.892-895.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J. A., Alexander M. Formation of nitrate from 3-nitropropionate by Aspergillus flavus. J Bacteriol. 1971 Feb;105(2):489–493. doi: 10.1128/jb.105.2.489-493.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. J., Bright H. J. Mechanism of oxidation of nitroethane by glucose oxidase. J Biol Chem. 1977 Jun 25;252(12):4361–4370. [PubMed] [Google Scholar]
- Porter D. J., Voet J. G., Bright H. J. Nitromethane. A novel substrate for D-amino acid oxidase. J Biol Chem. 1972 Mar 25;247(6):1951–1953. [PubMed] [Google Scholar]
- Sander J. Nitrosaminsynthese durch Bakterien. Hoppe Seylers Z Physiol Chem. 1968 Apr;349(4):429–432. [PubMed] [Google Scholar]
- Verstraete W., Alexander M. Mechanism of nitrification by Arthrobacter sp. J Bacteriol. 1972 Jun;110(3):962–967. doi: 10.1128/jb.110.3.962-967.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


