Abstract
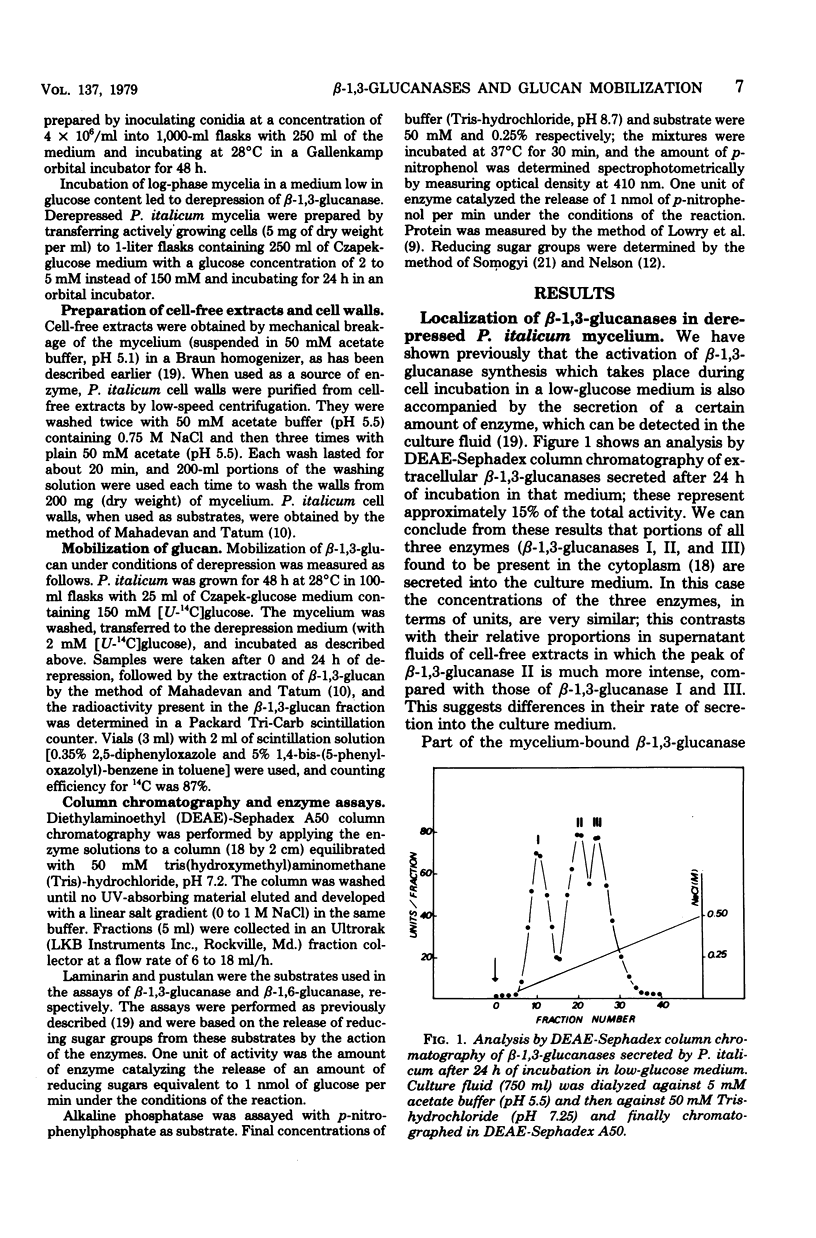
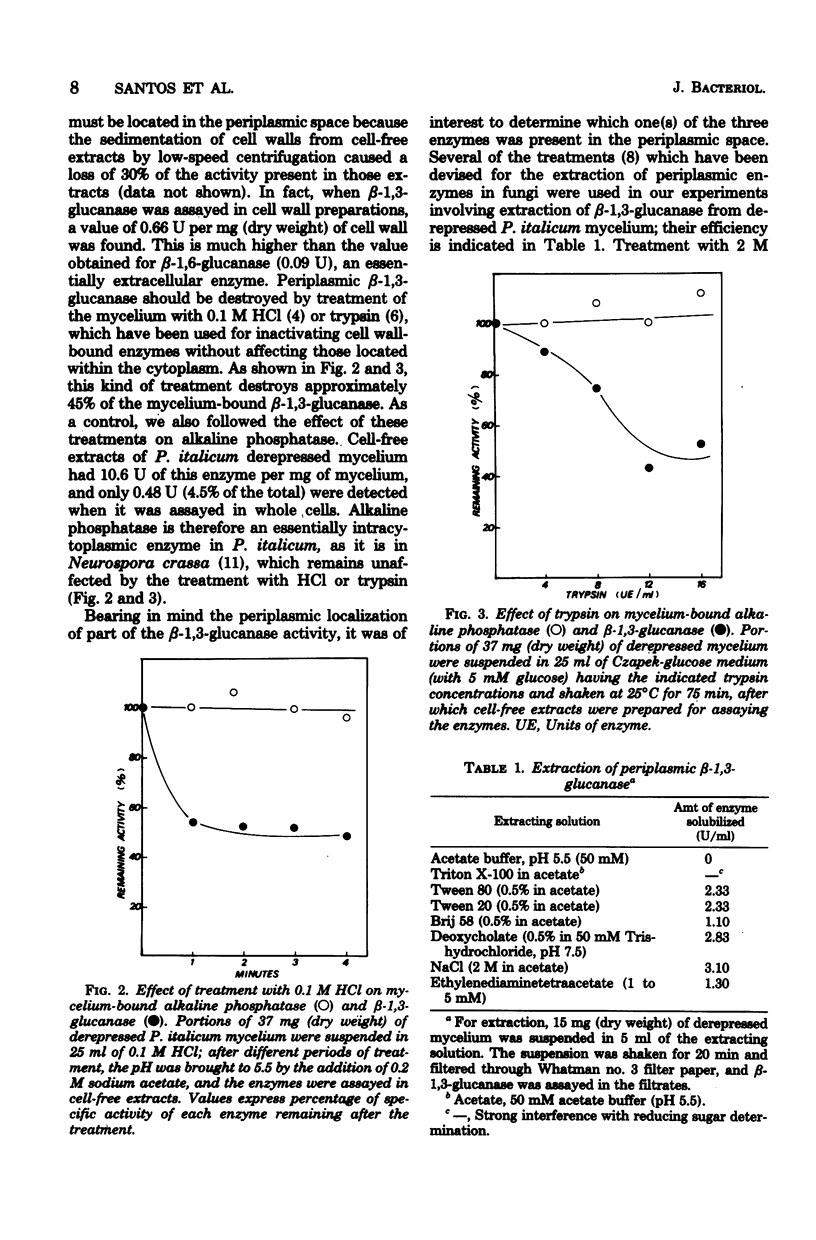
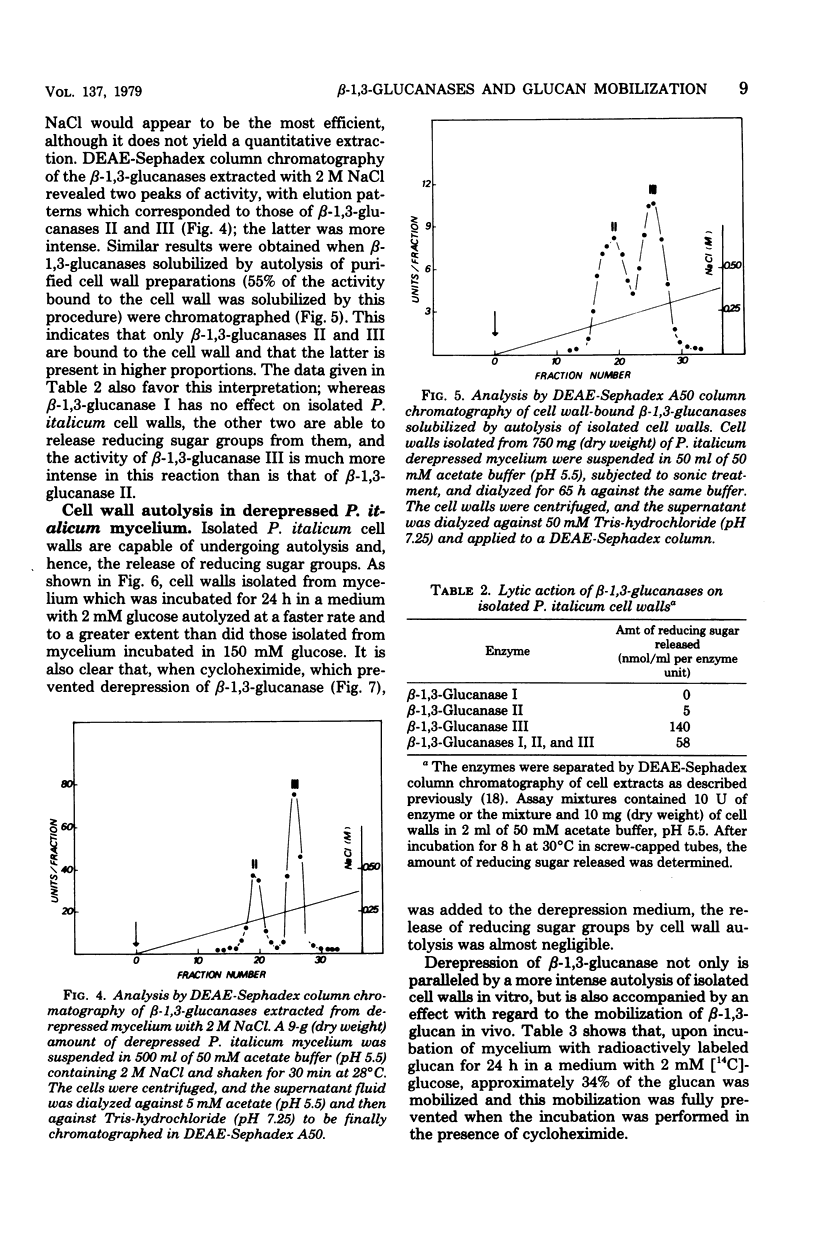
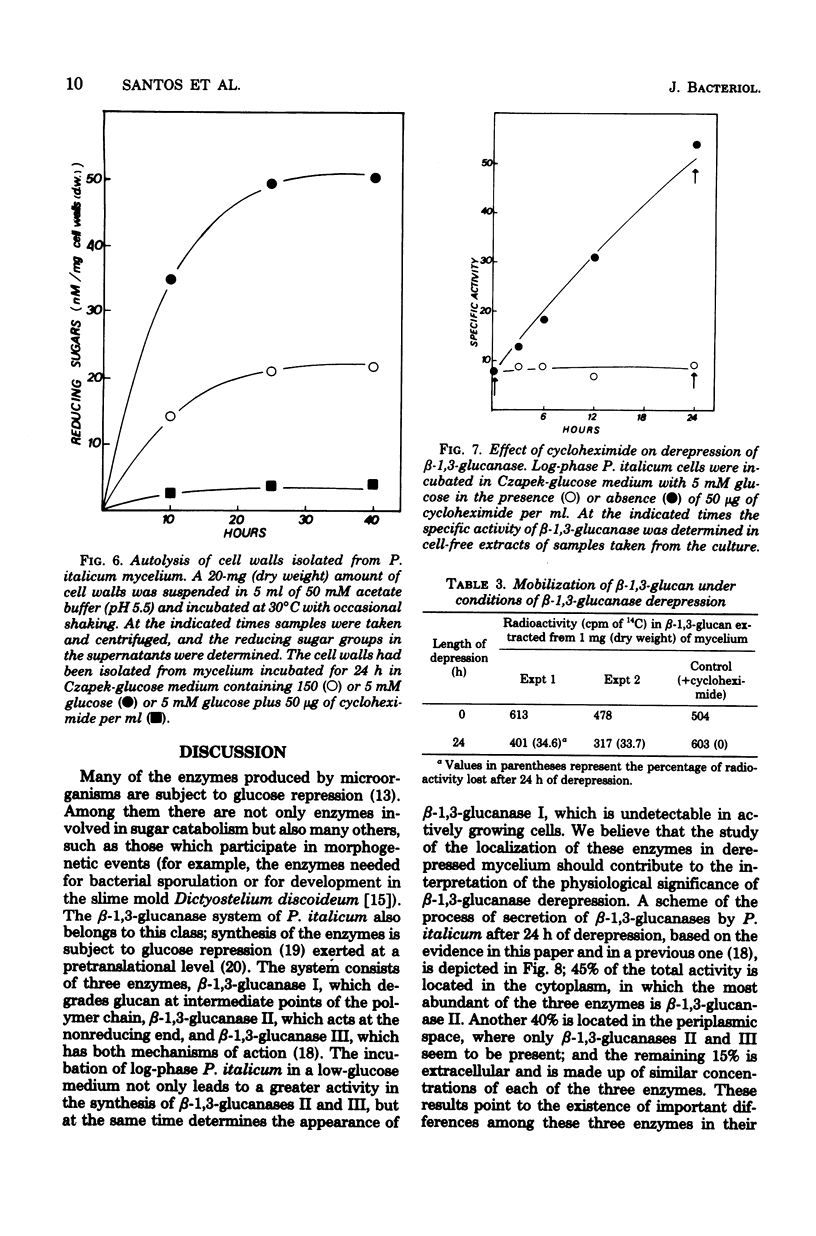
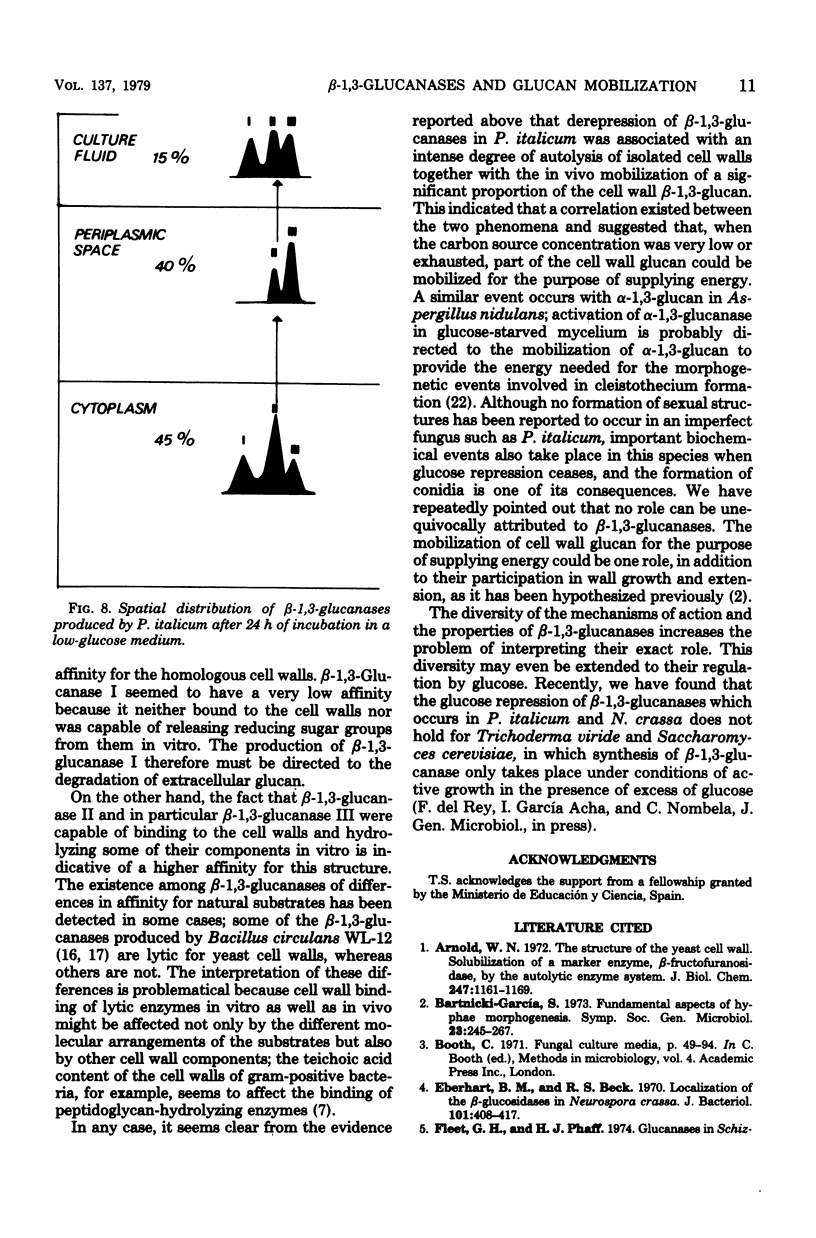
The localization of the derepressible beta-1,3-glucanases of Penicillium italicum and the cell wall autolysis under conditions of beta-1,3-glucanase derepression (24 h in a low-glucose medium) were studied. About 15% of the total activity was secreted into the culture medium during the 24-h period and consisted of similar amounts of each of the three beta-1,3-glucanases (I, II, III) produced by this species. Treatment of derepressed mycelia with periplasmic enzyme-inactivating agents resulted in a loss of 45% of the mycelium-bound beta-1,3-glucanase. Analysis of periplasmic enzymes solubilized by 2 M NaCl or by autolysis of isolated cell walls revealed that only beta-1,3-glucanases II and III were bound to the cell wall. These two enzymes were capable of releasing in vitro reducing sugars from cell walls, whereas beta-1,3-glucanase I was not. In addition, the autolytic activity of cell walls isolated from derepressed mycelium was greater than that of cell walls isolated from repressed mycelium. The incubation of the fungus in the low-glucose medium also resulted in the in vivo mobilization of 34% of the cell wall beta-1,3-glucan, and this mobilization was fully prevented by cycloheximide, which also blocked derepression of beta-1,3-glucanases. Derepression of beta-1,3-glucanase seems to be coupled to the mobilization of cell wall glucan.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N. The structure of the yeast cell wall. Solubilization of a marker enzyme, -fructofuranosidase, by the autolytic enzyme system. J Biol Chem. 1972 Feb 25;247(4):1161–1169. [PubMed] [Google Scholar]
- Eberhart B. M., Beck R. S. Localization of the beta-glucosidases in Neurospora crassa. J Bacteriol. 1970 Feb;101(2):408–417. doi: 10.1128/jb.101.2.408-417.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindsay B., Glaser L. Characterization of the N-acetylmuramic acid L-alanine amidase from Bacillus subtilis. J Bacteriol. 1976 Aug;127(2):803–811. doi: 10.1128/jb.127.2.803-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisker N., Katan J., Chet I., Henis Y. Release of cell-bound polygalacturonase and cellulase from mycelium of Rhizoctonia solani. Can J Microbiol. 1975 Apr;21(4):521–526. doi: 10.1139/m75-074. [DOI] [PubMed] [Google Scholar]
- Mahadevan P. R., Tatum E. L. Relationship of the major constituents of the Neurospora crassa cell wall to wild-type and colonial morphology. J Bacteriol. 1965 Oct;90(4):1073–1081. doi: 10.1128/jb.90.4.1073-1081.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf G. A. Regeneration of invertase in Neurospora crassa. J Bacteriol. 1973 Jul;115(1):146–152. doi: 10.1128/jb.115.1.146-152.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck Y., Rosengerger R. F. Autolytic enzymes in hyphae of Aspergillus nidulans: their action on old and newly formed walls. J Bacteriol. 1975 Jan;121(1):332–337. doi: 10.1128/jb.121.1.332-337.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Cailla H. L., Spitz E., Moran M. J., Rickenberg H. V. Effect of sugars on early biochemical events in development of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3183–3187. doi: 10.1073/pnas.73.9.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts F. M., Phaff H. J. Lysis of yeast cell walls. Lytic beta-(1 leads to 3)-glucanases from Bacillus circulans WL-12. Eur J Biochem. 1976 Mar 16;63(1):121–130. doi: 10.1111/j.1432-1033.1976.tb10214.x. [DOI] [PubMed] [Google Scholar]
- Rombouts F. M., Phaff H. J. Lysis of yeast cell walls. Lytic beta-(1 leads to 6)-glucanase from Bacillus circulans WL-12. Eur J Biochem. 1976 Mar 16;63(1):109–120. doi: 10.1111/j.1432-1033.1976.tb10213.x. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Santos T., Sanchez M., Villanueva J. R., Nombela C. Regulation of the beta-1,3-glucanase system in Penicillium italicum: glucose repression of the various enzymes. J Bacteriol. 1978 Feb;133(2):465–471. doi: 10.1128/jb.133.2.465-471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos T., Villanueva J. R., Nombela C. Production and catabolite repression of Penicillium italicum beta-glucanases. J Bacteriol. 1977 Jan;129(1):52–58. doi: 10.1128/jb.129.1.52-58.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos T., Villanueva J. R., Nombela C. Regulation of beta-1,3-glucanase synthesis in Penicillium italicum. J Bacteriol. 1978 Feb;133(2):542–548. doi: 10.1128/jb.133.2.542-548.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld B. J. Morphogenesis in Aspergillus nidulans. The significance of a alpha-1, 3-glucan of the cell wall and alpha-1, 3-glucanase for cleistothecium development. Biochim Biophys Acta. 1972 Jun 26;273(1):174–187. doi: 10.1016/0304-4165(72)90205-x. [DOI] [PubMed] [Google Scholar]


