Abstract
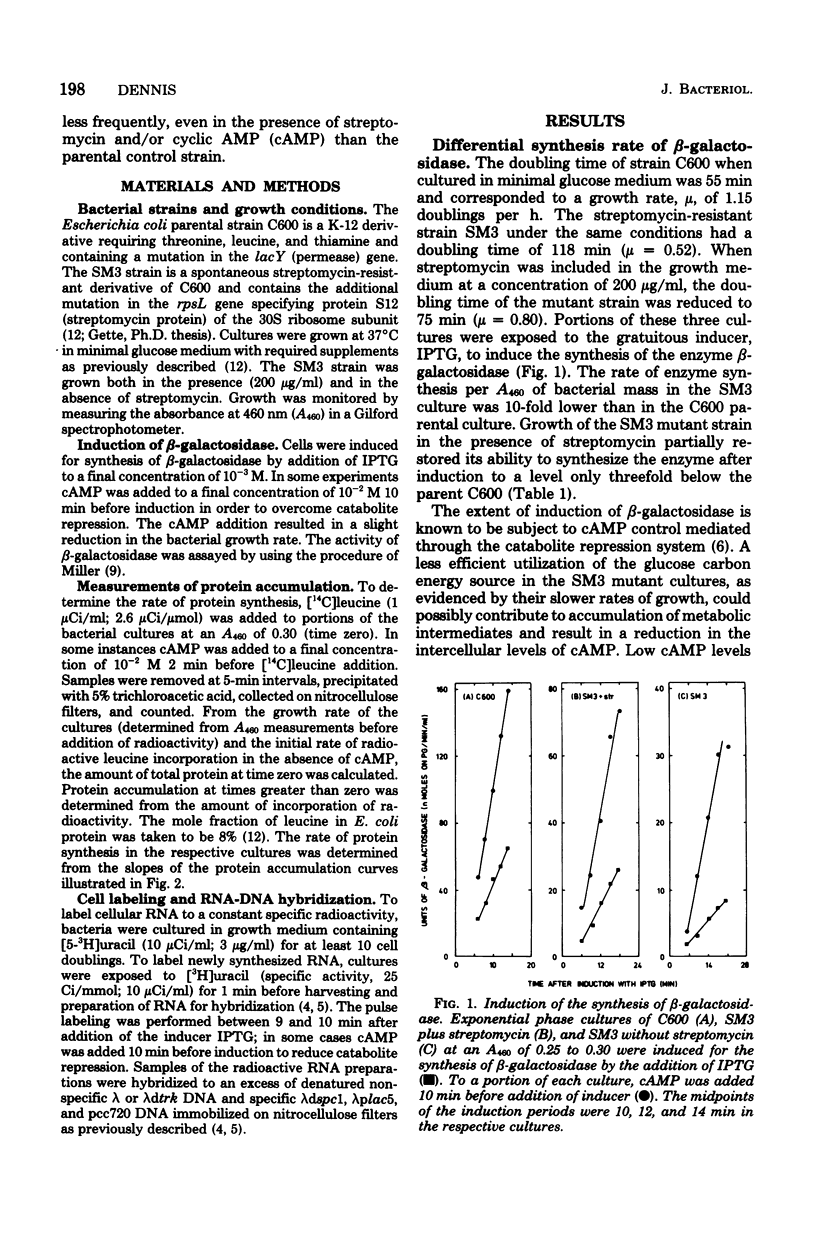
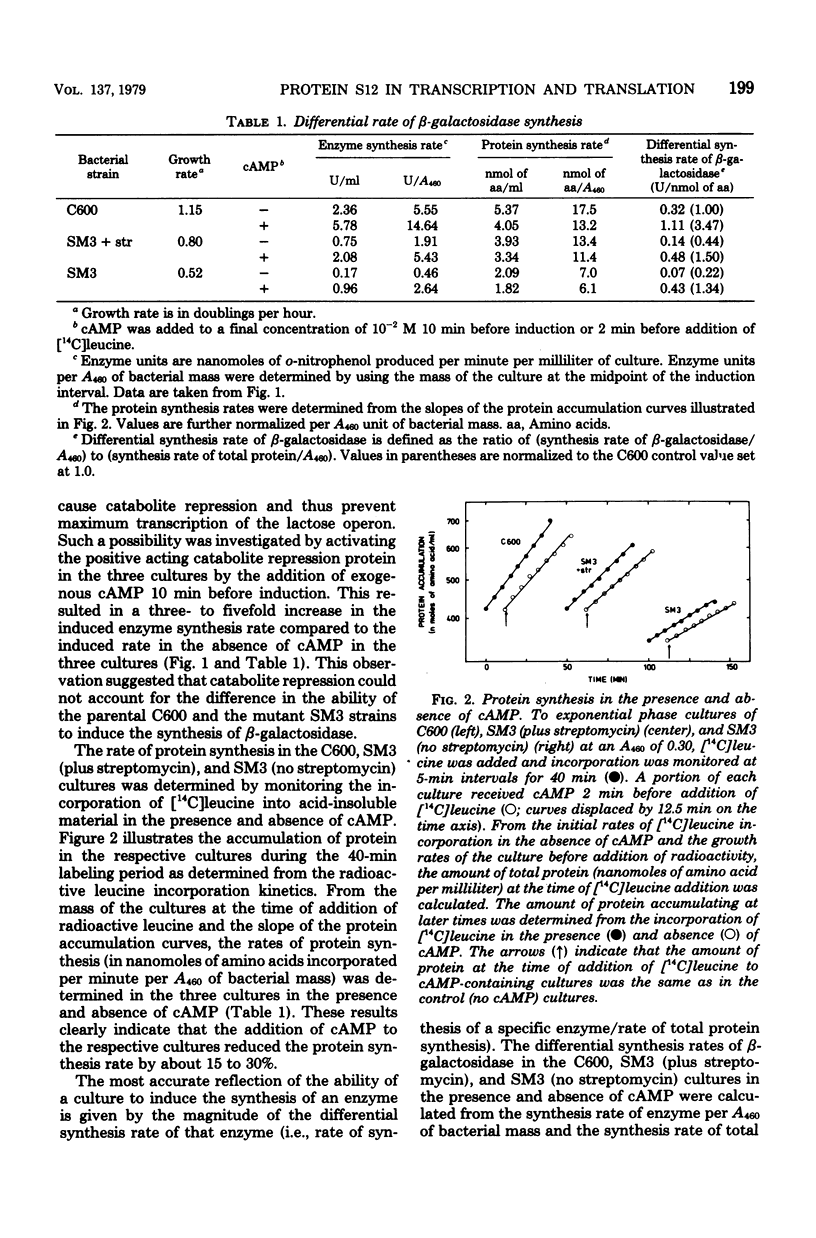
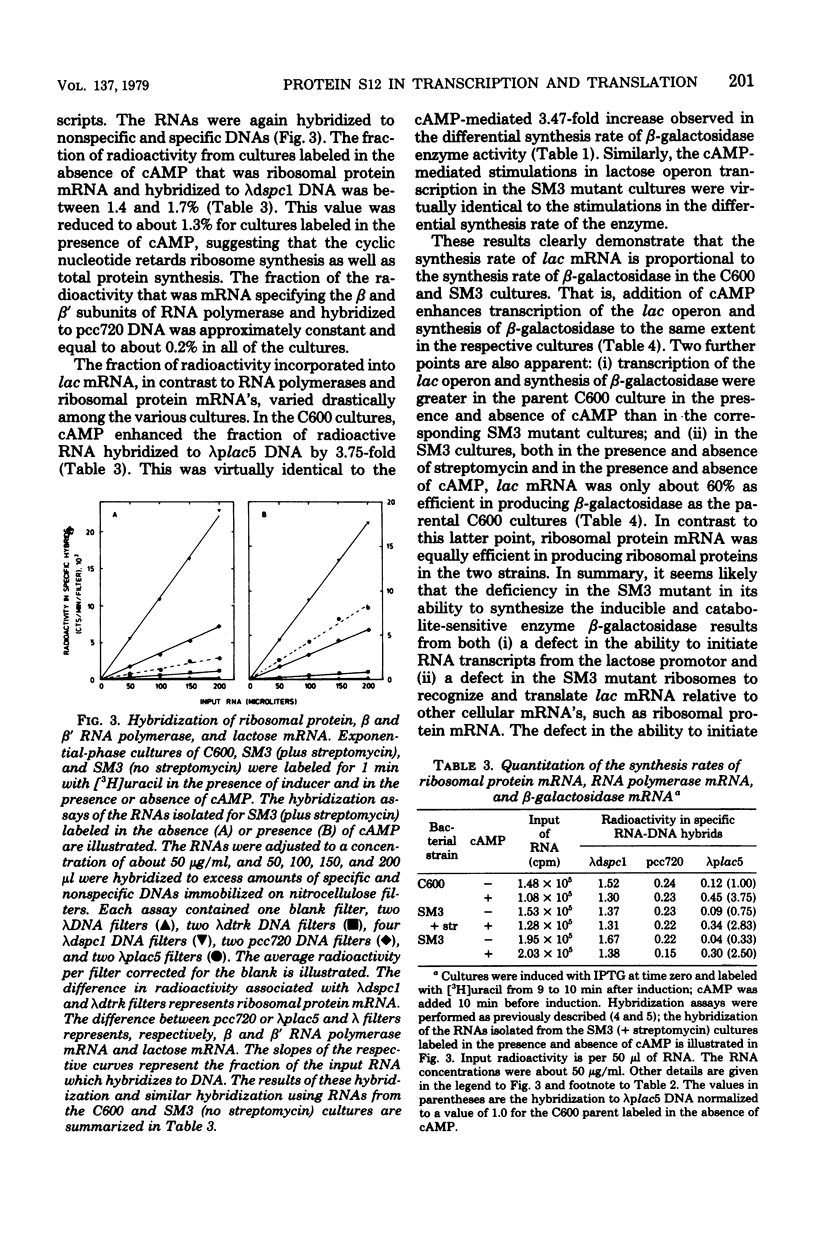
The role of the ribosomal protein S12 (streptomycin protein) in ribosome function and in other metabolic processes in the cell has been investigated. A spontaneous streptomycin-resistant strain of Escherichia coli (SM3) carrying a mutation in the rpsL gene is deficient in its ability to induce the synthesis of the enzyme bets-galactosidase. It was demonstrated that the reduced rate of enzyme synthesis results from deficiencies in both the transcription of the lactose operon and translation of the lactose operon mRNA. The transcription deficiency was in part due to increased catabolite repression and could therefore be partially suppressed by the addition of cyclic AMP. Streptomycin also appeared to partially suppress catabolite repression. In the SM3 mutant strain, the translation of the lactose operon mRNA was only about 60% as efficient as in the parental control, and addition of streptomycin did not alter the translation efficiency. In contrast, both transcription and translation of ribosomal protein mRNA were equally efficient in the two strains. These observations imply that mutational alterations in the ribosomal protein S12 either directly or indirectly alter (i) the extent of catabolite repression, (ii) the efficiency of transcription of the lactose operon even in the absence of catabolite repression, and (iii) the efficiency of translation of some but not all mRNA species in the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge E. A., Kurland C. G. Altered ribosomal protein in streptomycin-dependent Escherichia coli. Science. 1969 Dec 5;166(3910):1282–1284. doi: 10.1126/science.166.3910.1282. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. Interaction between mutations of ribosomes and RNA polymerase: a pair of strA and rif mutants individually temperature-insensitive but temperature-sensitive in combination. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1157–1161. doi: 10.1073/pnas.74.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coukell M. B., Polglase W. J. Relaxation of catabolite repression in streptomycin-dependent Escherichia coli. Biochem J. 1969 Feb;111(3):279–286. doi: 10.1042/bj1110279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Regulation of the expression of ribosomal protein genes in Escherichia coli. J Mol Biol. 1975 Sep 5;97(1):61–76. doi: 10.1016/s0022-2836(75)80022-2. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. Transcription patterns of adjacent segments on the chromosome of Escherichia coli containing genes coding for four 50S ribosomal proteins and the beta and beta' subunits of RNA polymerase. J Mol Biol. 1977 Oct 5;115(4):603–625. doi: 10.1016/0022-2836(77)90105-x. [DOI] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Schweiger M. Nitrofurans, a group of synthetic antibiotics, with a new mode of action: discrimination of specific messenger RNA classes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3386–3390. doi: 10.1073/pnas.73.10.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Young R., Dennis P. P., Nomura M. Role of ribosomal protein S12 in peptide chain elongation: analysis of pleiotropic, streptomycin-resistant mutants of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1320–1329. doi: 10.1128/jb.129.3.1320-1329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


