Abstract
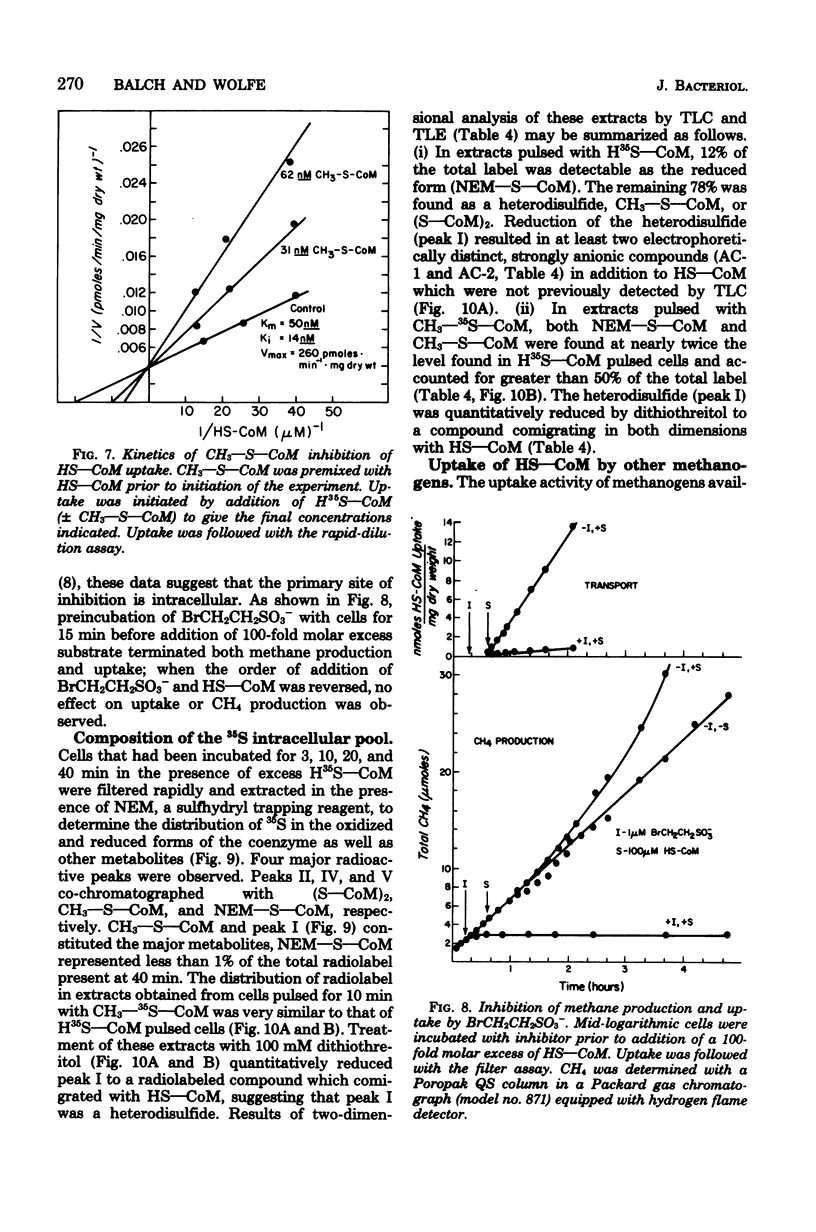
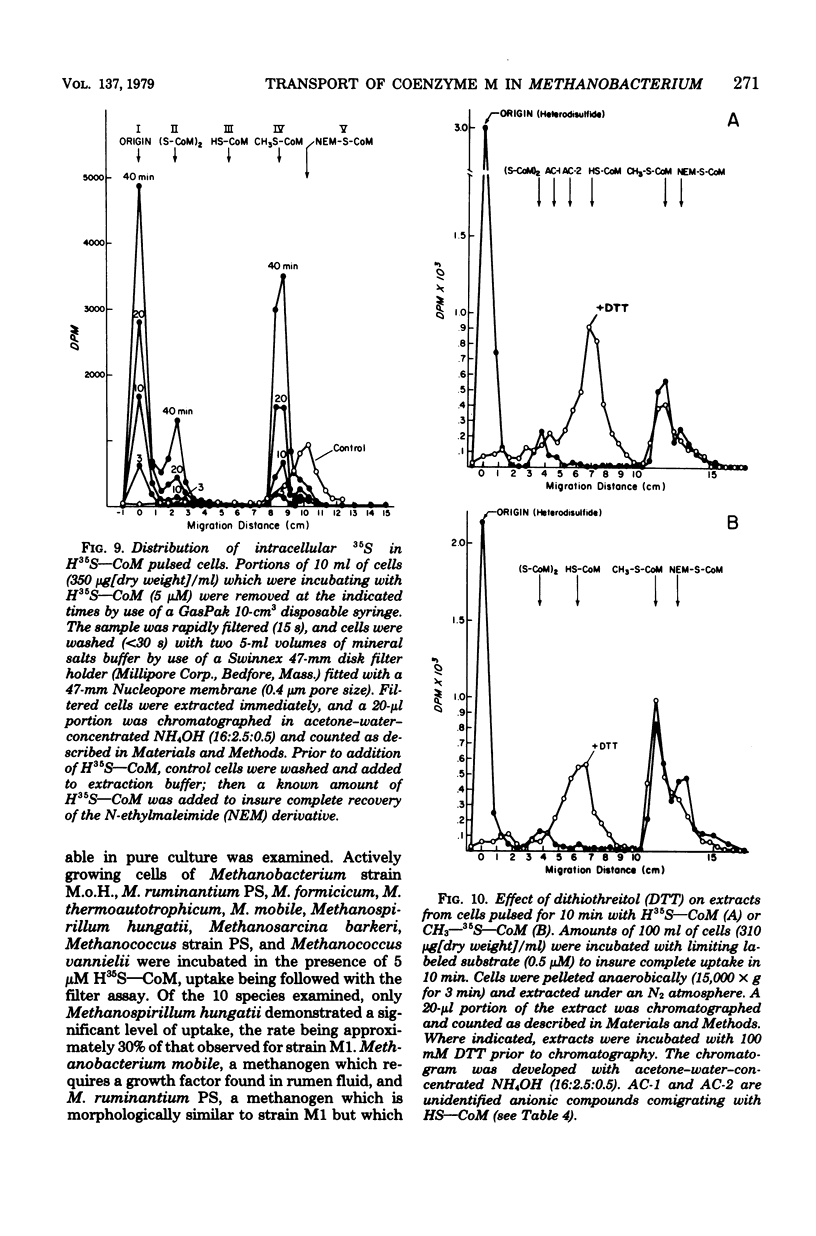
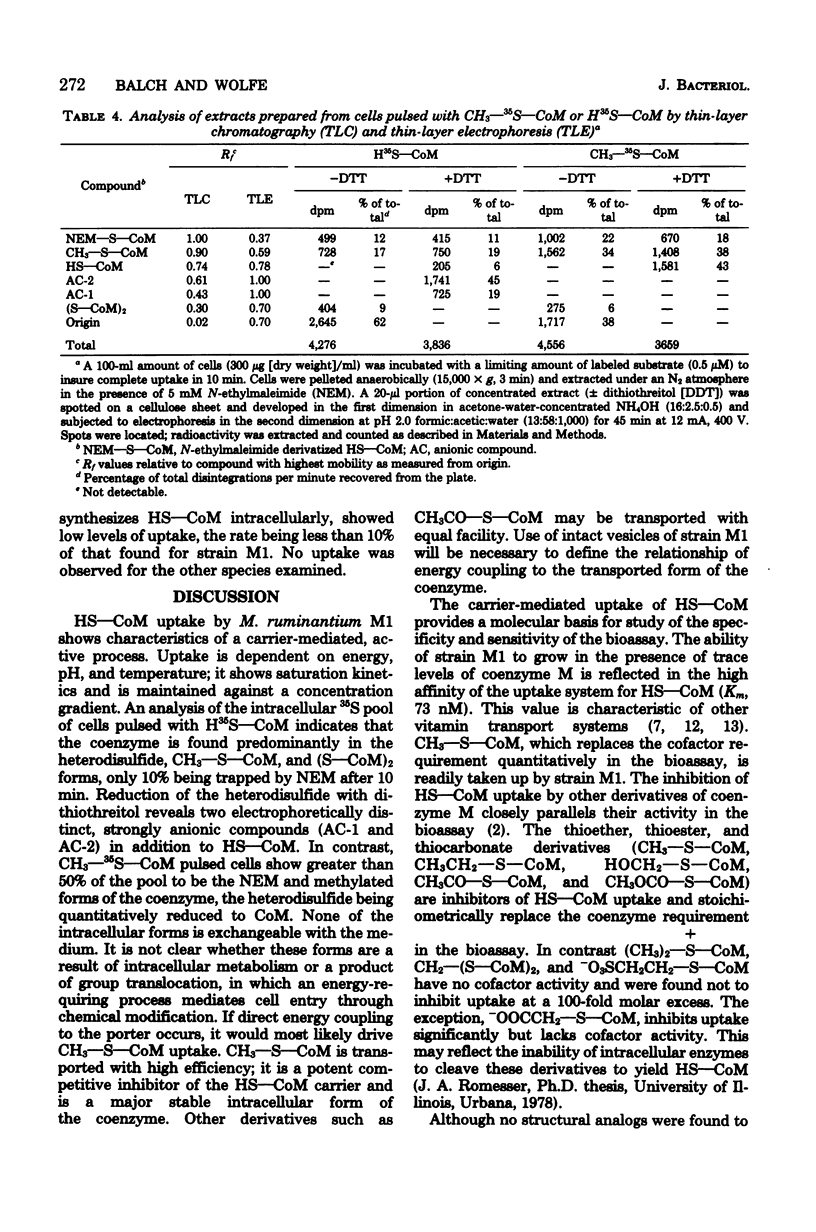
A system for transport of coenzyme M, 2-mercaptoethanesulfonic acid (HS--CoM), in Methanobacterium ruminatium strain M1 required energy, showed saturation kinetics, and concentrated the coenzyme against a gradient. The process was sensitive to temperature and was maximally active at pH 7.1. Cells took up HS--CoM at a linear rate, with a Vmax of 312 pmol/min per mg (dry weight) and an apparent Km of 73 nM. An intracellular pool of up to 5 mM accumulated which was not exchangeable with the medium. Uptake required both hydrogen and carbon dioxide; it was inhibited by O2. Bromoethanesulfonic acid (BrCH2CH2SO3-), a potent inhibitor of methanogenesis in cell-free extracts, inhibited both uptake and methane production. Results of inhibitor studies with derivatives and analogs of the coenzyme showed that the specificity of the carrier is restricted to a limited range of thioether, thioester, and thiocarbonate derivatives. 2-(Methylthio)ethanesulfonic acid (CH3--S--CoM) showed an apparent Ki for HS--CoM uptake of 15 nM, being taken up itself with a Vmax of 320 pmol/min per mg (dry weight) and an apparent Km of 50 nM. An analysis of intracellular pools after HS--CoM uptake indicated that the predominant forms are a heterodisulfide of unknown composition and CH3--S--CoM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Specificity and biological distribution of coenzyme M (2-mercaptoethanesulfonic acid). J Bacteriol. 1979 Jan;137(1):256–263. doi: 10.1128/jb.137.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C., Woodrow M. L. Transport of vitamin B12 in Escherichia coli: energy dependence. J Bacteriol. 1976 Oct;128(1):99–104. doi: 10.1128/jb.128.1.99-104.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., McBride B. C., Wolfe R. S. Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J Bacteriol. 1968 Mar;95(3):1118–1123. doi: 10.1128/jb.95.3.1118-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Girolamo P. M., Bradbeer C. Transport of vitamin B 12 in Escherichia coli. J Bacteriol. 1971 Jun;106(3):745–750. doi: 10.1128/jb.106.3.745-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958 Apr;74(2):443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Mulligan J. H., Snell E. E. Transport and metabolism of vitamin B6 in Salmonella typhimurium LT2. J Biol Chem. 1976 Feb 25;251(4):1052–1056. [PubMed] [Google Scholar]
- Mulligan J. H., Snell E. E. Transport and metabolism of vitamin B6 in lactic acid bacteria. J Biol Chem. 1977 Feb 10;252(3):835–839. [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane B., Snell E. E. Transport and metabolism of vitamin B6 in the yeast Saccharomyces carlsbergensis 4228. J Biol Chem. 1976 Feb 25;251(4):1042–1051. [PubMed] [Google Scholar]
- Shane B., Stokstad E. L. Transport and metabolism of folates by bacteria. J Biol Chem. 1975 Mar 25;250(6):2243–2253. [PubMed] [Google Scholar]
- Taylor C. D., McBride B. C., Wolfe R. S., Bryant M. P. Coenzyme M, essential for growth of a rumen strain of Methanobacterium ruminantium. J Bacteriol. 1974 Nov;120(2):974–975. doi: 10.1128/jb.120.2.974-975.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]


