Abstract
The production of new hippocampal neurons in adulthood has been well documented in rodents. Recent studies have extended these findings to other mammalian species, such as tree shrews and marmoset monkeys. However, hippocampal neurogenesis has not been demonstrated in adult Old World primates. To investigate this possibility, we injected 11 adult Old World monkeys of different ages (5–23 years) with the thymidine analog bromodeoxyuridine and examined the fate of the labeled cells at different survival times by using neuronal and glial markers. In the young-adult and middle-aged monkeys, we found a substantial number of cells that incorporated bromodeoxyuridine and exhibited morphological and biochemical characteristics of immature and mature neurons. New cells located in the dentate gyrus expressed a marker of immature granule neurons, Turned On After Division 64 kDa protein, as well as markers of mature granule neurons including neuron specific enolase, neuronal nuclei, and the calcium-binding protein calbindin. Fewer new cells expressed the astroglial marker glial fibrillary acidic protein. Evidence of neurogenesis was observed in the oldest monkeys (23 years) as well, but it appeared to be less robust. These results indicate that the adult brains of Old World monkeys produce new hippocampal neurons. Adult macaque monkeys may provide a useful primate model for studying the functional significance of adult neurogenesis.
A hallmark of many neurodegenerative diseases in humans is the permanent loss of neurons. The human brain is generally believed incapable of producing new neurons to repair damaged areas in adulthood. The key to stimulating neurogenesis in damaged brain regions may lie in studying the systems that continue to produce new neurons throughout life. In adult birds, extensive neurogenesis has been observed in both the song system and in the hippocampal formation (1–3). In rodents, the production of new neurons in adulthood has been reported in two brain regions, the hippocampal formation and the olfactory bulb (4–8). Recent evidence has shown that two other mammalian species, tree shrews and marmoset monkeys, produce hippocampal granule neurons in adulthood (9, 10), and most recently, this phenomenon has been demonstrated in humans (11). Although the existence of this process is intriguing, the function of new neurons in the adult brain remains enigmatic. However, there is no Old World primate animal model in which to study adult neurogenesis. Indeed, previous studies using 3H-thymidine autoradiography reported no neurogenesis in the brains of adult Old World monkeys (12, 13). We reexamined this possibility by using bromodeoxyuridine, a thymidine analog, and report here that a substantial number of hippocampal neurons are indeed produced in the brains of adult Old World monkeys.
MATERIALS AND METHODS
Animal Treatments.
Adult male and female macaque monkeys from Princeton University and the German Primate Center were used for these studies. To determine whether new neurons are produced in the brains of adult Old World monkeys, we injected adult macaque monkeys (Macaca fascicularis and Macaca mulatta) with bromodeoxyuridine (BrdUrd; 75 mg/kg 1–5 intraperitoneal injections; see Table 1) and examined the brains with combined immunocytochemistry for BrdUrd and cell-specific markers at various survival times (2 hr to 2 weeks after the last injection). BrdUrd is a thymidine analog that labels proliferating cells and their progeny (14). The 2-hr survival time (n = 5) was used to investigate the location of proliferating cells in the adult brain. Two hours is sufficient for BrdUrd uptake into cells in S phase but not for completion of mitosis or migration. Other schedules of BrdUrd injection (three to five injections with 1- to 2-week survival times after the last injection) were used to maximize the chance of observing new cells that expressed neuronal markers and minimize the likelihood that new cells would die in the interval between labeling and perfusion. In the rat, newly generated cells express neuronal markers between 1 and 3 weeks after mitosis (5). All animal experimentation was approved by the Princeton Institutional Animal Care committee and the Government of Lower Saxony, Germany and was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals used in this study were sexually mature adults. M. fascicularis reach sexual maturity at ≈4 years (males and females) and reach maximal body size by 6 years (females) and 9 years (males) (15). M. fascicularis older than 16 years are considered aged (16). Thus, monkeys that were sexually mature but had not reached their maximum body size were considered young adults and those beyond the period of maximal body growth but not aged were considered middle-aged. In addition, two adult M. mulatta monkeys were examined for purposes of comparison. These monkeys were sexually mature and completely grown but not aged (see Table 1). At the end of each survival time, the monkeys were deeply anesthetized with sodium pentobarbital (Nembutal; 100 mg/kg) and were transcardially perfused by using 4.0% paraformaldehyde in 0.1 M phosphate buffer.
Table 1.
Characteristics of experimental animals and numbers of BrdUrd-labeled cells in the dentate gyrus
| No. | Species | Age | Sex | No. of BrdUrd injections | Survival time | No. of BrdUrdlabeled cells |
|---|---|---|---|---|---|---|
| 1 | M. fascicularis | 5 (YA) | M | 5 | 2 weeks | 4,308 |
| 2 | M. fascicularis | 5 (YA) | M | 1 | 2 hr | 3,288 |
| 3 | M. fascicularis | 7 (MA) | F | 5 | 2 weeks | 1,968 |
| 4 | M. fascicularis | 10 (MA) | M | 3 | 1 week | 2,748 |
| 5 | M. mulatta | 10 (MA) | F | 5 | 2 weeks | 1,896 |
| 6 | M. mulatta | 12 (MA) | F | 5 | 2 weeks | 1,176 |
| 7 | M. fascicularis | 15 (MA) | F | 3 | 2 weeks | 780 |
| 8 | M. fascicularis | 16 (MA) | M | 1 | 2 hr | 876 |
| 9 | M. fascicularis | 23 (A) | M | 1 | 2 hr | 420 |
| 10 | M. fascicularis | 23 (A) | M | 1 | 2 hr | 336 |
| 11 | M. fascicularis | 23 (A) | M | 1 | 2 hr | 228 |
Histological Procedures.
The brains were removed from the cranial cavities, and brain sections were cut either coronally through the entire hippocampal formation or sagittally through the forebrain by using an oscillating tissue slicer. Every 12th section (40 μm thick) throughout the hippocampal formation was saved for stereological analysis and was processed immunohistochemically for BrdUrd combined with one of several cell-specific markers. The sections were processed for BrdUrd labeling by using both peroxidase and fluorescent techniques.
For BrdUrd peroxidase staining, the tissue first was treated by heating in 0.1 M citric acid buffer (pH 6.0) followed by incubation in hydrogen peroxide, trypsin, and 2 M HCl, blocked in normal horse serum and incubated in mouse monoclonal antibody to BrdUrd (Novocastra, Newcastle, U.K.; 1:250). The sections then were processed by using a Vectastain Elite ABC kit (Vector Laboratories) followed by nickel ammonium sulfate-enhanced diaminobenzidine. The sections were subsequently incubated in primary antibodies to a marker of immature neurons (17, 18), Turned-On-After-Division 64-kDa protein (TOAD-64, rabbit anti-TOAD-64, 1:20,000; gift of S. Hockfield, Yale University); also used were markers of mature granule neurons (19), including the calcium-binding protein calbindin of 28 kDa (rabbit anti-calbindin, 1:2,500; Chemicon), neuron-specific enolase (NSE) (rabbit anti-NSE, 1:5000; Polysciences), or the astroglial marker glial fibrillary acidic protein (goat anti-glial fibrillary acidic protein, 1:1,000; Santa Cruz Biotechnology) using a Vectastain Elite ABC kit with a brown diaminobenzidine reaction. The sections then were counterstained for Nissl by using either cresyl violet or neutral red. For fluorescence immunocytochemistry, the tissue was pretreated for BrdUrd staining followed by incubation in mouse monoclonal anti-BrdUrd (1:250, NovoCastra) or rat monoclonal anti-BrdUrd (1:200, Accurate Scientific, Westbury, NY). Subsequently, the sections were incubated in biotinylated anti-mouse followed by Avidin-Neutralite-Cascade Blue (1:500, Molecular Probes) or anti-rat IgG followed by streptavidin-Alexa 568 (1:1,000, Molecular Probes) for visualization of BrdUrd incorporation. After several rinses in PBS, BrdUrd-labeled sections were incubated in one of the primary antisera of the cell-specific markers listed above or the neuronal marker neuronal nuclei (NeuN) (mouse anti-NeuN, 1:100, Chemicon). This tissue then was reacted with goat anti-rabbit-Alexa 488 (1:500, Molecular Probes), rabbit anti-goat-CY2 (1:100, Jackson ImmunoResearch), or goat anti-mouse Alexa 488 (1:1,000, Molecular Probes) was rinsed and dried, and then was coverslipped under 3:1 glycerol:PBS. Sections stained for TOAD-64 alone were counterstained with the DNA dye Hoechst 44323. Control sections were processed as described above with omission of the primary antisera. Tissue stained for fluorescence was viewed with an Olympus (New Hyde Park, NY) BX-60 fluorescent microscope and then with a confocal scanning laser microscope (Zeiss 510 LSM) for verification of double labeling.
Data Analysis.
A modified version of the stereological optical fractionator method (20) was performed on slides that were coded before quantitative analyses. For every 12th section through the dentate gyrus, the number of labeled cells was determined by using an Olympus BX-60 light microscope with an Optronics International (Chelmsford, MA) color video camera connected to a Dell OptiPlex computer (Round Rock, TX). Every labeled cell was counted excluding those in the outermost focal plane to avoid counting cell caps. The percentage of BrdUrd-labeled cells that were immunoreactive for cell-specific markers was determined from a series of every 12th section throughout the dentate gyrus of each animal. Stereological estimates were made of the total number of each cell type located within structures that had BrdUrd-labeled cells.
RESULTS
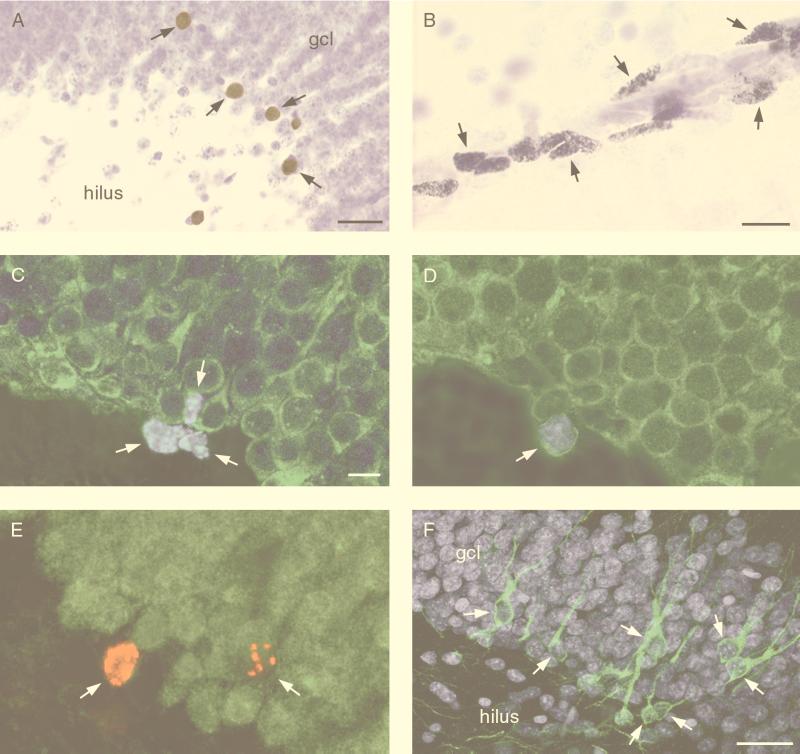
In all monkeys examined (Table 1), BrdUrd-labeled cells were observed in the dentate gyrus (Fig. 1A), in the subventricular zone lining the wall of the lateral ventricle, and in a region corresponding to the rostral migratory stream (Fig. 1B) described for rodents (7, 8). In monkeys that received injections of BrdUrd >2 hr before perfusion (n = 6), BrdUrd-labeled mitotic figures were occasionally observed in the rostral migratory stream, dentate gyrus, and subventricular zone. In the hippocampal formation, BrdUrd-labeled cells were observed primarily in the dentate gyrus, and, at all levels examined, clusters of 2–5 labeled cells were observed in the hilus or the subgranular zone, the region between the granule cell layer and the hilus (Fig. 1C).
Figure 1.
Photomicrographs (A and B) and confocal laser scanning microscopic images (C–F) depicting new cells produced in the adult monkey brain. BrdUrd-labeled cells were observed in the granule cell layer of the dentate gyrus (A) (arrows) (animal 7) and in a region corresponding to the rostral migratory stream in rodents (B) (arrows) (animal 4). (C) Clusters of BrdUrd-labeled cells (blue nuclear stain) were observed in the subgranular zone of the dentate gyrus (arrows) (animal 2). Many of these clustered cells in animals injected with BrdUrd followed by short survival times (2 hr after BrdUrd injection) were not immunoreactive for NSE (green cytoplasmic stain). (D) In monkeys perfused 1–2 weeks after the last BrdUrd injection, BrdUrd-labeled cells expressing the neuronal marker NSE were observed (arrow is an example from animal 3). (E) BrdUrd-labeled cells in the granule cell layer of animals perfused 2 weeks after the last of five BrdUrd injections were immunoreactive for NeuN (arrows indicate double-labeled cells) (animal 1). (F) TOAD-64 stained cells with the morphology of granule cells were observed in the granule cell layer (arrows) (animal 1) (granule cells stained here with the DNA dye Hoechst 44323) gcl, granule cell layer. [Bars = 30 μm (A), = 15 μm (B), = 10 μm (C, D, and E), and = 40 μm (F).]
Because of the relatively low number of animals, it was not possible to perform a parametric study of the number of BrdUrd-labeled cells at various survival times. However, despite variable treatment regimens, clear differences in the number of BrdUrd-labeled cells in the dentate gyrus were observed among the different age groups. In general, younger monkeys exhibited more BrdUrd-labeled cells in the dentate gyrus than older monkeys. In the monkeys that received a single BrdUrd injection and were perfused 2 hr later (n = 5), BrdUrd-labeled cells in the dentate gyrus were more numerous in the young adult monkey (age 5 years) compared with the middle aged and older monkeys (age 23 years) treated similarly (Table 1).
In animals that were injected with BrdUrd and were perfused 1–2 weeks after the first injection (n = 6), labeled cells were observed in the deep aspect of the granule cell layer of the dentate gyrus (Fig. 1 d and e). The numbers of BrdUrd-labeled cells in the dentate gyrus of animals that received multiple BrdUrd injections with longer survival times (n = 6) ranged from a high of 4,308 to a low of 780. The highest number of BrdUrd-labeled cells in the dentate gyrus was observed in a young adult monkey (animal 1) that received multiple BrdUrd injections (five BrdUrd injections, 2-week postinjection survival time). Even the youngest animal included in the middle-aged group (animal 3) that received multiple BrdUrd injections (five BrdUrd injections, 2-week postinjection survival time) had noticeably fewer BrdUrd-labeled cells in the dentate gyrus (1, 968 BrdUrd-labeled cells) compared with the young adult monkey treated similarly (animal 1). In the animals with survival times of at least 1 week after the last BrdUrd injection (n = 6), many of the labeled cells exhibited morphological characteristics of granule neurons, i.e., round or oval medium sized cell bodies (Fig. 1 a, d, and e). These cells did not express the glial marker glial fibrillary acidic protein, and many were immunoreactive for markers of immature and mature granule neurons, including TOAD-64, calbindin, NSE and NeuN (Fig. 1 d and e). For animals injected five times with BrdUrd (animals 1, 3, 5, and 6), the average number of BrdUrd-labeled cells in the dentate gyrus that expressed these markers was substantial (BrdUrd-labeled TOAD-64-positive cells, 1,230.5 ± 436.9; BrdUrd-labeled NSE-positive cells, 822.1 ± 345.9; BrdUrd-labeled calbindin-positive cells, 793.2 ± 232.9).
Throughout the dentate gyrus, cells labeled with TOAD-64, a marker of immature neurons, were observed. These cells exhibited morphological characteristics of granule neurons, including dendrites that extended through the molecular layer (Fig. 1f). Previous studies have demonstrated that cells labeled with TOAD-64 or the embryonic form of neural cell adhesin molecule (polysialylated neural cell adhesion molecule) in the dentate gyrus of adult rats are adult-generated granule neurons (18, 21). Our observation of BrdUrd-labeled TOAD-64-positive cells in monkeys with time >2 hr after BrdUrd injection is consistent with the view that these cells are newly generated neurons. Stereological analyses revealed decreases in the number of TOAD-64-expressing cells in the granule cell layer with advancing age; although young adult monkeys exhibited a substantial number of TOAD-64 labeled cells, there were fewer present in the middle-aged group and only an occasional labeled cell in the old monkeys [F(2, 5) = 154.2, P < 0.01] (Table 2). No obvious differences were observed in the distribution of BrdUrd- or TOAD-64-labeled cells between M. fascicularis and M. mulatta of the same age group (Table 2).
Table 2.
Numbers of TOAD-64-labeled granule cells in the dentate gyrus at different ages
| Species | Young Adult | Middle Aged | Aged |
|---|---|---|---|
| M. fascicularis | 7,002.0 ± 305.7 (n = 4) | 1,976.0 ± 293.7 (n = 4) | 126 ± 114.0 (n = 2)* |
| M. mulatta | 3,738.5 ± 516.5 (n = 2) |
These data represent stereological estimates of the total number of TOAD-64-labeled cells in the dentate gyrus in M. fascicularis and M. mulatta monkeys described in Table 1.
Significant difference from young adult and middle-aged monkeys; P < 0.05; one-way ANOVA followed by Tukey post hoc comparisons.
DISCUSSION
These results indicate that the production of a substantial number of new neurons continues into adulthood in the dentate gyrus of two species of macaque monkeys. In addition, our observation of BrdUrd-labeled and TOAD-64-labeled cells in the rostral migratory stream presents the possibility that new neurons are added to the olfactory bulb in adult monkeys. The presence of cells with granule neuron morphology that stain for TOAD-64, a marker of immature neurons, as well as BrdUrd-labeled cells that express TOAD-64, calbindin, NSE, and NeuN in the granule cell layer, supports the view that new neurons are being formed in the dentate gyrus of adult monkeys.
In addition to the present findings, hippocampal neuron production in adulthood has been reported to occur in a variety of vertebrate species (2, 4–6, 9, 10). Using similar techniques of BrdUrd labeling and stereological analyses, we have detected evidence of substantial neurogenesis in the dentate gyrus of many mammalian species, including mice, rats, tree shrews, marmoset monkeys, and macaque monkeys. The recent observation of granule cell genesis in the dentate gyrus of adult humans (11) further indicates that this phenomenon is common to most, if not all, mammalian species.
Our findings are at variance with previously published papers that reported no neuron production in adult macaque monkeys by using 3H-thymidine autoradiography (12, 13). There may be several reasons these studies did not detect neuron production in the adult monkey brain. The most compelling is that the post-3H-thymidine-injection survival times were, for the most part, very long. Survival times of >1 year were used in many cases, thus presenting the possibility that labeled cells may have become undetectable after multiple cell divisions. Alternatively, in animals with long survival times, cells that incorporated 3H-thymidine divided and differentiated into neurons might have degenerated in the interval before perfusion. Indeed, many new hippocampal neurons appear to die in adult laboratory rats within several weeks of DNA synthesis (5, 22).
If some of the hippocampal neurons produced in the adult monkey die shortly after their production, then the challenge will be to determine how the survival of these new neurons might be promoted. A previous study demonstrated that birds living in the wild have a greater number of adult-generated hippocampal neurons than those living in captivity (2). Likewise, mice living in “enriched environment” conditions maintain more new granule neurons than those living in standard laboratory cages (2, 6). These findings suggest that a more complex environment may rescue new neurons from death. However, multiple variables, including food, stress, and social interaction, differ between captivity and wild living in the case of birds and between standard laboratory cage and enriched environment living in the case of mice (2, 6). Barnea and Nottebohm (2) have shown a relationship between spatial behavior and the number of adult-generated hippocampal neurons. In black-capped chickadees, more adult-generated hippocampal neurons are present, presumably as a result of enhanced cell survival, during the fall, when these animals engage in seed-storing behavior (2). Given the putative role of the hippocampal formation in spatial learning (23, 24), these data suggest that engaging in behaviors that require the hippocampus may prevent new neurons from dying. Indeed, recent studies have shown that hippocampal-dependent learning specifically, and not learning that does not require the hippocampus or generalized experience in the absence of overt learning, increases the number of adult-generated granule neurons in the rat, presumably by preventing cell death (22).
Studies performed in several mammalian species have indicated that stressful experiences prevent the proliferation of granule cell precursors in adulthood (9, 10). Previous findings demonstrating an inhibitory effect of glucocorticoids on the production of new granule neurons (25) suggest that the stress-induced decrease in neurogenesis may be mediated by glucocorticoids. Basal levels of circulating glucocorticoids have been shown to increase throughout life in both rats and humans (26), presenting the possibility that age-related decline in hippocampal neurogenesis is the result of elevated glucocorticoid levels. These findings imply that the potential for hippocampal neurogenesis in the aging brain may be restored by controlling glucocorticoid levels.
The persistence of hippocampal neurogenesis in the adult brain, and its conservation across evolution, suggests that these neurons have an important function. The vast literature linking the hippocampal formation with learning (23, 24, 27, 28) presents the possibility that these adult-generated neurons may participate in the formation of new memories, a suggestion first proposed by Nottebohm for song learning in birds (29). The continual renewal of new hippocampal neurons may underlie, in part, the transient role the hippocampal formation seems to play in learning and memory (28).
Acknowledgments
This work was supported by National Institutes of Health Grants MH52423 and EY11347.
ABBREVIATIONS
- BrdUrd
bromodeoxyuridine
- TOAD-64
Turned-On-After-Division 64-kDa protein
- NeuN
neuronal nuclei
- NSE
neuronspecific enolase
References
- 1.Alvarez-Buylla A, Theelen M, Nottebohm F. Proc Natl Acad Sci USA. 1988;85:8722–8726. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnea A, Nottebohm F. Proc Natl Acad Sci USA. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnea A, Nottebohm F. Proc Natl Acad Sci USA. 1996;93:714–717. doi: 10.1073/pnas.93.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altman J, Das G D. J Comp Neurol. 1965;124:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 5.Cameron H A, Woolley C S, McEwen B S, Gould E. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 6.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 7.Rousselot P, Lois C, Alvarez-Buylla A. J Comp Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- 8.Doetsch F, Garcia-Verdugo J M, Alvarez-Buylla A. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould E, McEwen B S, Tanapat P, Galea L A M, Fuchs E. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould E, Tanapat P, McEwen B S, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3768–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson P S, Perfilieva E, Bjork-Eriksson T, Alborn A M, Nordborg C, Peterson D A, Gage F H. Nat Med. 1998;11:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 12.Rakic P. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 13.Eckenhoff M F, Rakic P. J Neurosci. 1988;8:2729–2747. doi: 10.1523/JNEUROSCI.08-08-02729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowakowski R S, Lewin S B, Miller M W. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 15.Napier J R, Napier P H. A Handbook of Living Primates. New York: Academic; 1967. p. 218. [Google Scholar]
- 16.Veenema H C, Spruijt B M, Gispen W H, van Hooff J A. Neurobiol Aging. 1997;5:509–515. doi: 10.1016/s0197-4580(97)00107-3. [DOI] [PubMed] [Google Scholar]
- 17.Minturn J E, Geschwind D H, Fryer H J, Hockfield S. J Comp Neurol. 1995;355:369–379. doi: 10.1002/cne.903550304. [DOI] [PubMed] [Google Scholar]
- 18.Parent J M, Yu T W, Leibowitz R T, Geschwind D H, Sloviter R S, Lowenstein D H. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould E, Tanapat P. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 20.West M J, Slomianka L, Gundersen H J G. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 21.Seki T, Arai Y. J Neurosci. 1993;13:2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould E, Beylin A, Tanapat P, Reeves A J, Shors T J. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 23.Jarrard L E. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 24.Wallenstein G V, Eichenbaum H, Hasselmo M E. Trends Neurosci. 1998;21:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 25.Cameron H A, Gould E. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 26.Sapolsky R M, Krey L C, McEwen B S. Neurobiol Aging. 1986;7:331–335. doi: 10.1016/0197-4580(86)90159-4. [DOI] [PubMed] [Google Scholar]
- 27.Clark R E, Squire L R. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 28.Squire L R, Zola S M. Philos Trans R Soc London B. 1997;352:1663–1673. doi: 10.1098/rstb.1997.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nottebohm F. In: Neural Control of Reproductive Function. Lakoski J, Perez-Polo J R, Rassin D, editors. New York: Liss; 1989. pp. 583–601. [Google Scholar]