Abstract
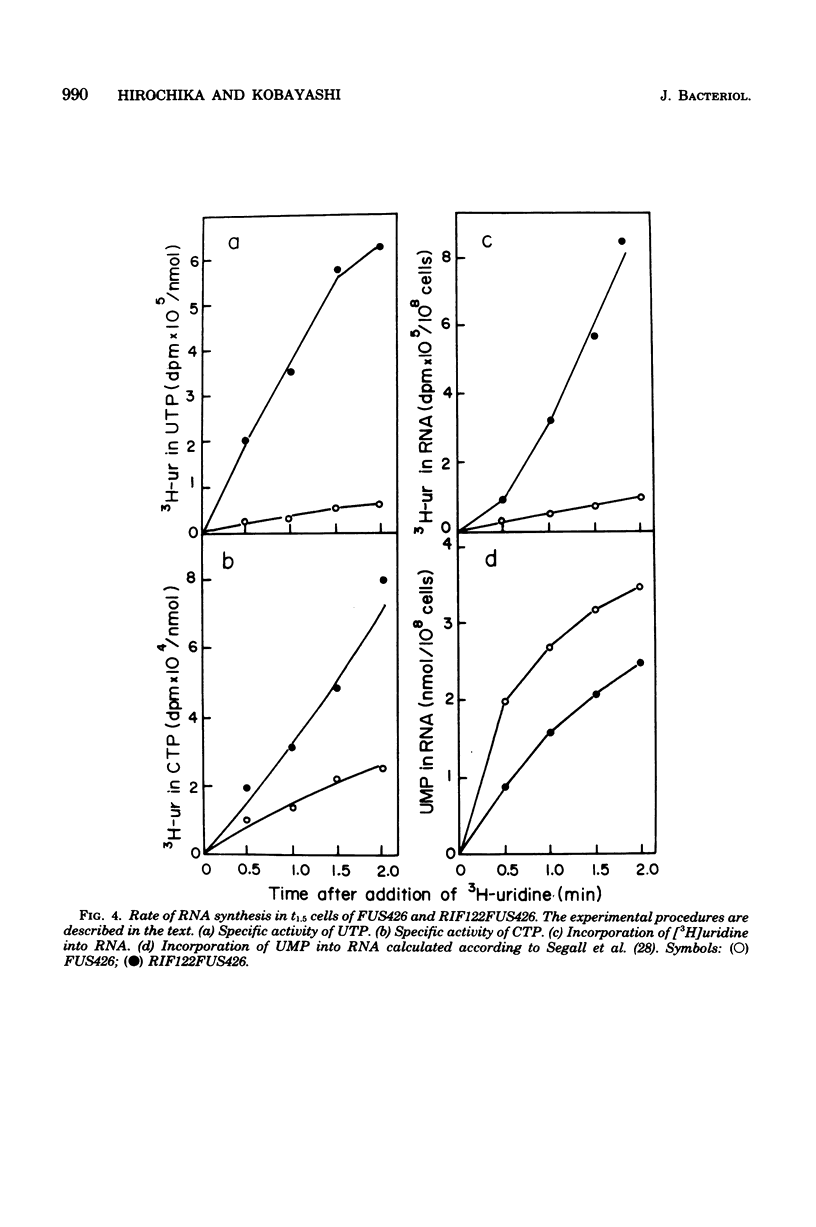
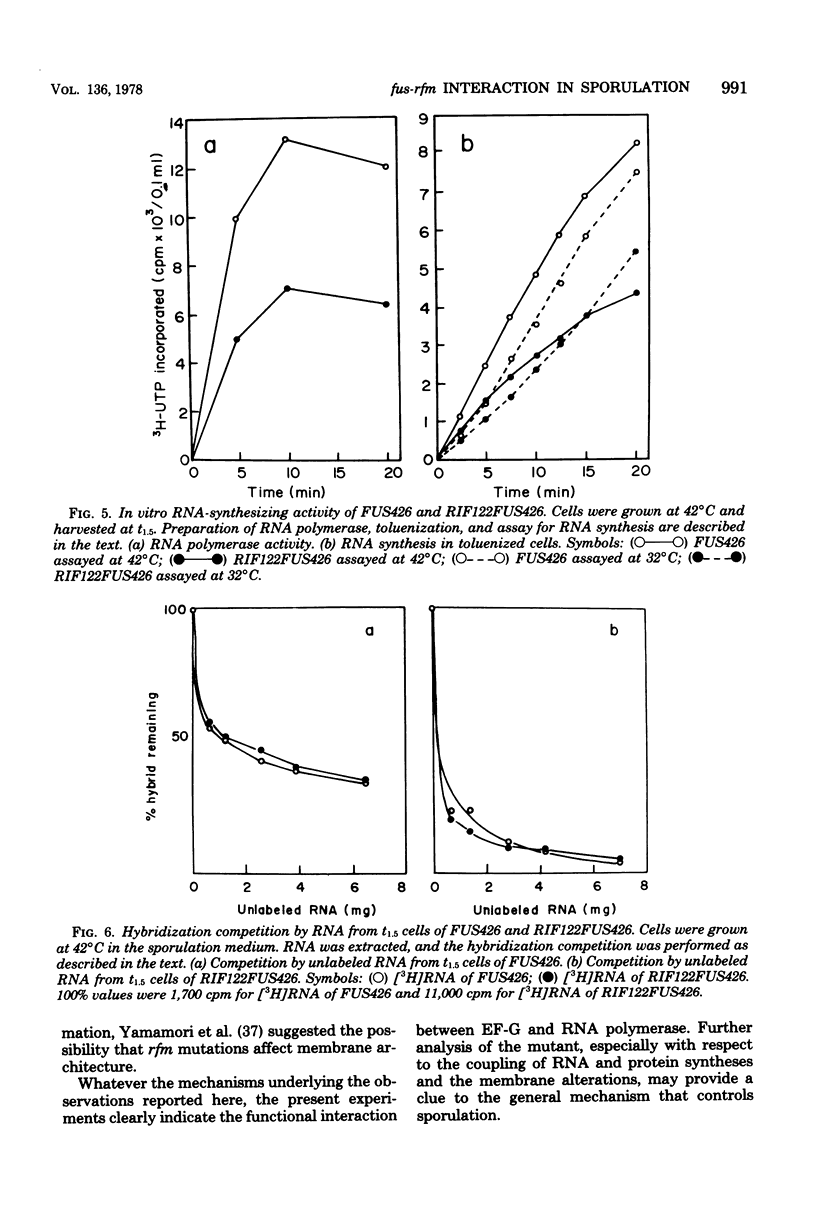
A class of rifampin-resistant (rfm) mutations of Bacillus subtilis suppresses the temperature-sensitive sporulation of a fusidic acid-resistant mutant, FUS426, which has an altered elongation factor G. The rfm mutation suppressed only the sporulation defect caused by the elongation factor G mutation, but could not suppress other types of induced sporulation defects. Genetic and biochemical analyses showed that the sporulation suppression by the rfm mutation was caused by a single mutation in RNA polymerase. After the early sporulation phase, the apparent rate of RNA synthesis of FUS426, measured by [3H]uracil or [3H]uridine incorporation into RNA, became lower than that of the wild-type strain, and this decrease was reversed by the rfm mutation. However, when the total rate of RNA synthesis of FUS426 was calculated by measuring the specific activity of [3H]UTP and [3H]CTP, it was higher than that of the rfm mutant, RIF122FUS426. The possible mechanism of the functional interaction between elongation factor G and RNA polymerase during sporulation is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohin J. P., Rigomier D., Schaeffer P. Ethanol sensitivity of sporulation in Bacillus subtilis: a new tool for the analysis of the sporulation process. J Bacteriol. 1976 Aug;127(2):934–940. doi: 10.1128/jb.127.2.934-940.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. Interaction between mutations of ribosomes and RNA polymerase: a pair of strA and rif mutants individually temperature-insensitive but temperature-sensitive in combination. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1157–1161. doi: 10.1073/pnas.74.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCioccio R. A., Strauss N. Patterns of transcription in Bacillus subtilis during sporulation. J Mol Biol. 1973 Jun 25;77(2):325–336. doi: 10.1016/0022-2836(73)90338-0. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Bergmann R. Alteration of the ribosomal fraction of Bacillus subtilis during sporulation. Biochim Biophys Acta. 1973 Feb 23;299(1):136–141. doi: 10.1016/0005-2787(73)90404-8. [DOI] [PubMed] [Google Scholar]
- Graham R. S., Bott K. F. Antibiotic-resistant mutants of Bacillus subtilis conditional for sporulation. Mol Gen Genet. 1975;137(3):227–237. doi: 10.1007/BF00333018. [DOI] [PubMed] [Google Scholar]
- Gross C., Engbaek F., Flammang T., Burgess R. Rapid micromethod for the purification of Escherichia coli ribonucleic acid polymerase and the preparation of bacterial extracts active in ribonucleic acid synthesis. J Bacteriol. 1976 Oct;128(1):382–389. doi: 10.1128/jb.128.1.382-389.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Burtis K. C., Doi R. H. Reconstitution studies show that rifampicin resistance is determined by the largest polypeptide of Bacillus subtilis RNA polymerase. J Biol Chem. 1977 Dec 25;252(24):9024–9031. [PubMed] [Google Scholar]
- Harford N., Sueoka N. Chromosomal location of antibiotic resistance markers in Bacillus subtilis. J Mol Biol. 1970 Jul 28;51(2):267–286. doi: 10.1016/0022-2836(70)90142-7. [DOI] [PubMed] [Google Scholar]
- Kaplan S., Atherly A. G., Barrett A. Synthesis of stable RNA in stringent Escherichia coli cells in the absence of charged transfer RNA. Proc Natl Acad Sci U S A. 1973 Mar;70(3):689–692. doi: 10.1073/pnas.70.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Muto A., Osawa S. Control of stable RNA synthesis in a temperature-sensitive mutant of elongation factor G of Bacillus subtilis. Mol Gen Genet. 1974 May 31;130(3):203–214. doi: 10.1007/BF00268800. [DOI] [PubMed] [Google Scholar]
- Kimura A. Regulation of ribonucleic acid synthesis in spheroplasts, cold-shocked cells, and toluene-treated cells of Escherichia coli. J Bacteriol. 1976 Oct;128(1):123–129. doi: 10.1128/jb.128.1.123-129.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Kobayashi K., Kobayashi Y. Isolation and characterization of fusidic acid-resistant, sporulation-defective mutants of Bacillus subtilis. J Bacteriol. 1977 Oct;132(1):262–269. doi: 10.1128/jb.132.1.262-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. Sporulation-specific translational discrimination in Bacillus subtilis. J Mol Biol. 1974 Jul 15;86(4):855–863. doi: 10.1016/0022-2836(74)90358-1. [DOI] [PubMed] [Google Scholar]
- Linn T., Greenleaf A. L., Losick R. RNA polymerase from sporulating Bacillus subtilis. Purification and properties of a modified form of the enzyme containing two sporulation polypeptides. J Biol Chem. 1975 Dec 25;250(24):9256–9261. [PubMed] [Google Scholar]
- Linn T., Losick R., Sonenshein A. L. Rifampin resistance mutation of Bacillus subtilis altering the electrophoretic mobility of the beta subunit of ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1387–1390. doi: 10.1128/jb.122.3.1387-1390.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Ulbrich N. Specific binding of formylated initiator-tRNA to Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3064–3067. doi: 10.1073/pnas.73.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portalier R., Worcel A. Association of the folded chromosome with the cell envelope of E. coli: characterization of the proteins at the DNA-membrane attachment site. Cell. 1976 Jun;8(2):245–255. doi: 10.1016/0092-8674(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Rhaese H. J., Groscurth R. Studies on the control of development. In vitro synthesis of HPN and MS nucleotides by ribosomes from either sporulating or vegetative cells of Bacillus subtilis. FEBS Lett. 1974 Aug 15;44(1):87–93. doi: 10.1016/0014-5793(74)80312-1. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Segall J., Tjian R., Pero J., Losick R. Chloramphenicol restores sigma factor activity to sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4860–4863. doi: 10.1073/pnas.71.12.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Doi R. H. Transcription from the complementary deoxyribonucleic acid strands of Bacillus subtilis during various stages of sporulation. J Bacteriol. 1974 Feb;117(2):775–782. doi: 10.1128/jb.117.2.775-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C. A simple method for extraction of RNA from E. coli utilizing diethyl pyrocarbonate. Anal Biochem. 1970 Feb;33(2):459–463. doi: 10.1016/0003-2697(70)90316-7. [DOI] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. Recognition properties of the beta subunit of Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1976 Oct;128(1):264–270. doi: 10.1128/jb.128.1.264-270.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Johnson C. W., Ginther C. L., Leighton T., Wittmann H. G. Erythromycin resistant mutations in Bacillus subtilis cause temperature sensitive sporulation. Mol Gen Genet. 1977 Jan 18;150(2):147–159. doi: 10.1007/BF00695395. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow R. M., Lazzarini R. A. The rates of synthesis and chain elongation of ribonucleic acid in Escherichia coli. J Biol Chem. 1969 Mar 10;244(5):1128–1136. [PubMed] [Google Scholar]
- Yamamori T., Ito K., Yura T., Suzuki T., Iino T. Ribonucleic acid polymerase mutant of Escherichia coli defective in flagella formation. J Bacteriol. 1977 Oct;132(1):254–261. doi: 10.1128/jb.132.1.254-261.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


