Abstract
We demonstrate that tandem duplications of the histidine transport operon can be selected by requesting elevated levels of transport activity to be present. Several strains were constructed which contain duplications heterozygotic for either hisJ, hisQ, or hisP. The size of one duplication which was analyzed in detail is about 16 genes, with one end close to the promoter site (dhuA) of the histidine transport operon and, therefore, enclosing about 12 more genes counterclockwise to this operon. Duplication-carrying strains could be utilized for the selection of deletion mutations by requiring both copies of the operon to be rendered defective simultaneously and, therefore, unable to transport into the cell an inhibitory histidine analog, alpha-hydrazino imidazole propionic acid. Over 60% (probably as high as 100%) of the alpha-hydrazino imidazole propionic acid-resistant strains arising in the selection are deletion mutants. The principle of our selection method is generally applicable and will be useful in the accumulation of deletions for mapping and fusing of genes and other purposes.
Full text
PDF




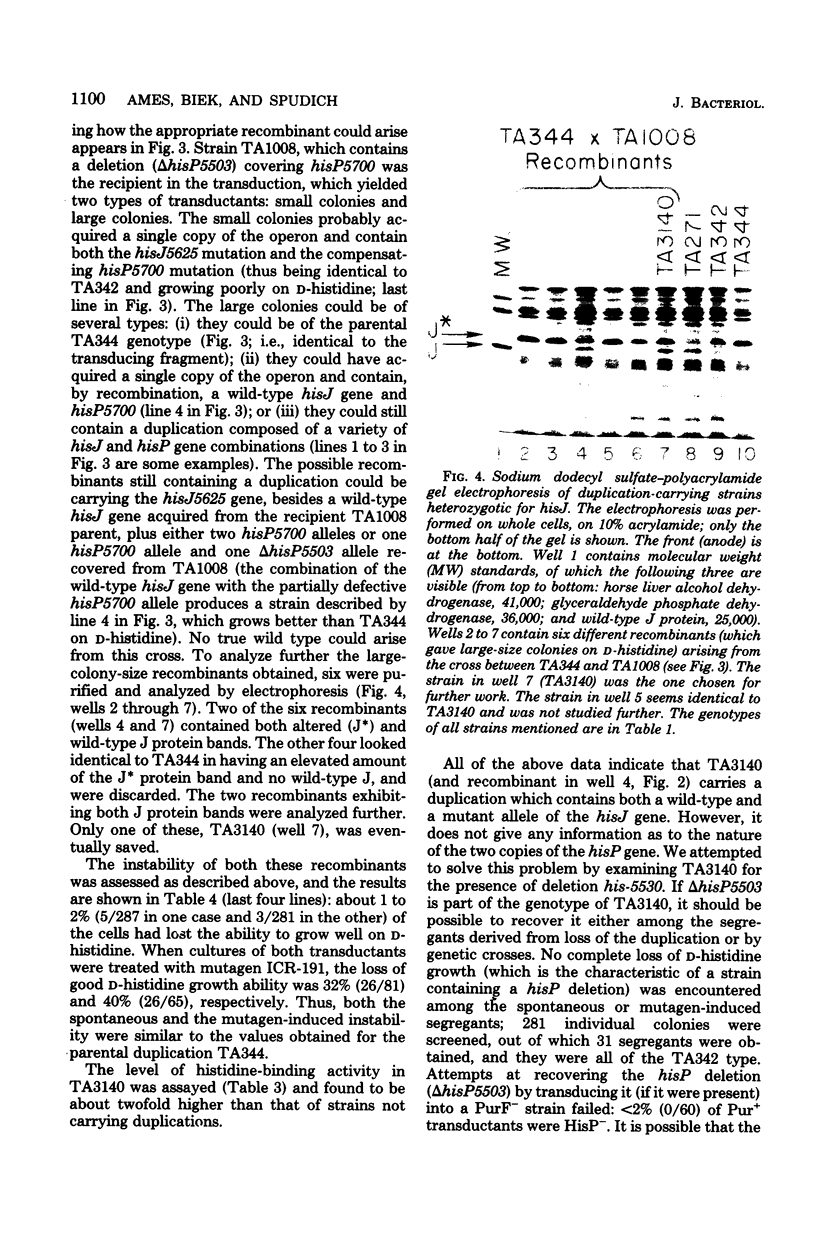








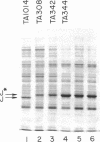
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Whitfield H. J., Jr Frameshift mutagenesis in Salmonella. Cold Spring Harb Symp Quant Biol. 1966;31:221–225. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Noel K. D., Taber H., Spudich E. N., Nikaido K., Afong J. Fine-structure map of the histidine transport genes in Salmonella typhimurium. J Bacteriol. 1977 Mar;129(3):1289–1297. doi: 10.1128/jb.129.3.1289-1297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Ames G. F., Roth J. R. Histidine and aromatic permeases of Salmonella typhimurim. J Bacteriol. 1968 Nov;96(5):1742–1749. doi: 10.1128/jb.96.5.1742-1749.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spurich E. N. Protein-protein interaction in transport: periplasmic histidine-binding protein J interacts with P protein. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1877–1881. doi: 10.1073/pnas.73.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Combriato G. Genetic duplications induced at very high frequency by ultraviolet irradiation in Escherichia coli. Mol Gen Genet. 1973 Dec 31;127(3):197–214. doi: 10.1007/BF00333760. [DOI] [PubMed] [Google Scholar]
- Kemper J. Gene order and co-transduction in the leu-ara-fol-pyrA region of the Salmonella typhimurium linkage map. J Bacteriol. 1974 Jan;117(1):94–99. doi: 10.1128/jb.117.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Walczak W., Klopotowski T. Mutants of Salmonella typhimurium able to utilize D-histidine as a source of L-histidine. J Bacteriol. 1971 Jan;105(1):28–37. doi: 10.1128/jb.105.1.28-37.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S. G., Ames G. F. The histidine-binding protein J, a histidine transport component, has two different functional sites. J Biol Chem. 1974 Nov 10;249(21):6976–6983. [PubMed] [Google Scholar]
- Lever J. E. Purification and properties of a component of histidine transport in Salmonella typhimurium. The histidine-binding protein J. J Biol Chem. 1972 Jul 10;247(13):4317–4326. [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. A method for detection of phage mutants with altered transducing ability. Mol Gen Genet. 1971;110(4):378–381. doi: 10.1007/BF00438281. [DOI] [PubMed] [Google Scholar]
- Sletzinger M., Firestone R. A., Reinhold D. F., Rooney C. S., Nicholson W. H. The alpha-hydrazino analog of histidine. J Med Chem. 1968 Mar;11(2):261–263. doi: 10.1021/jm00308a015. [DOI] [PubMed] [Google Scholar]
- Straus D. S., D'Ari Straus L. Large overlapping tandem genetic duplications in Salmonella typhimurium. J Mol Biol. 1976 May 5;103(1):143–153. doi: 10.1016/0022-2836(76)90056-5. [DOI] [PubMed] [Google Scholar]
- Straus D. S. Induction by mutagens of tandem gene duplications in the glyS region of the Escherichia coli chromosome. Genetics. 1974 Nov;78(3):823–830. doi: 10.1093/genetics/78.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]