Abstract
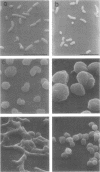
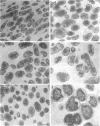
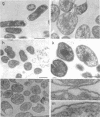
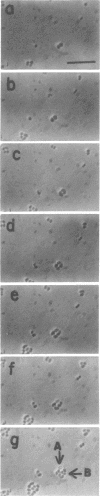
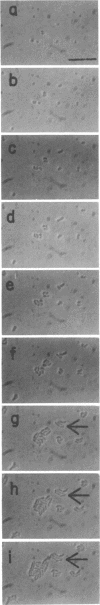
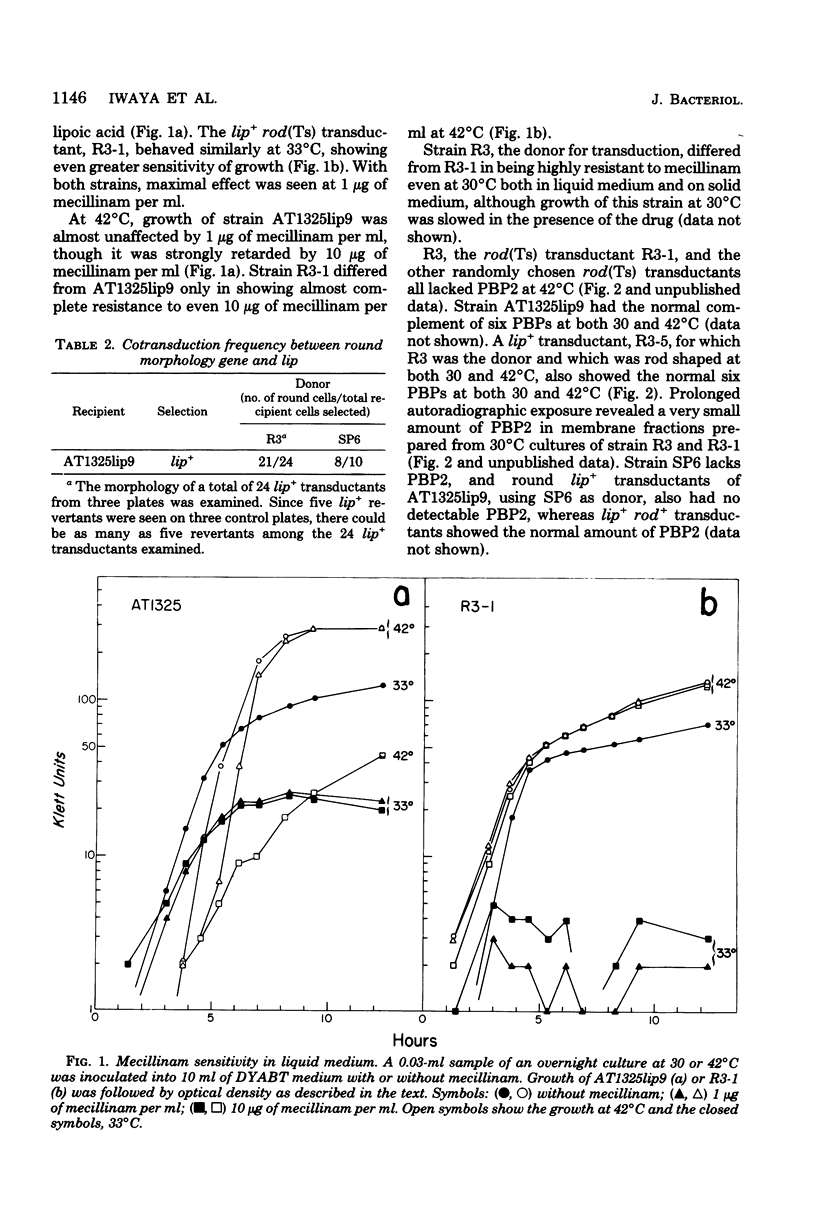
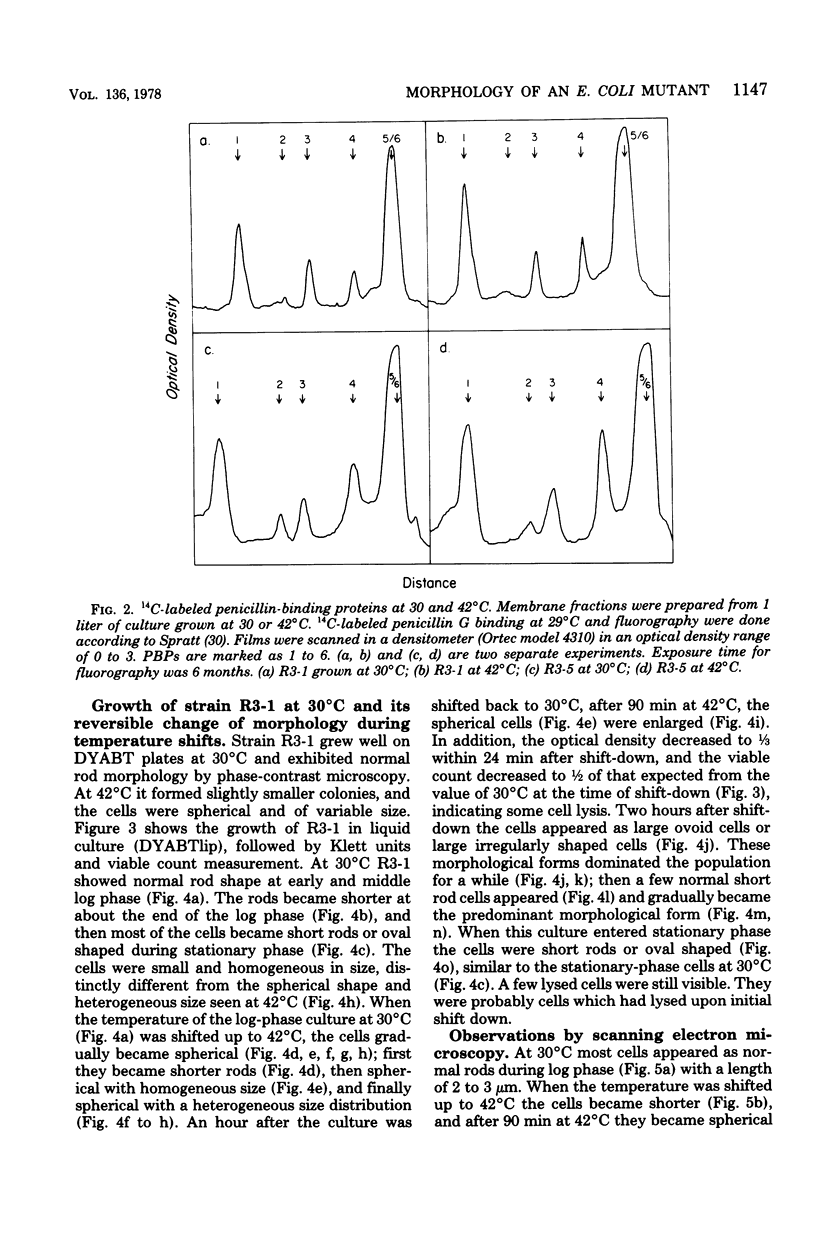
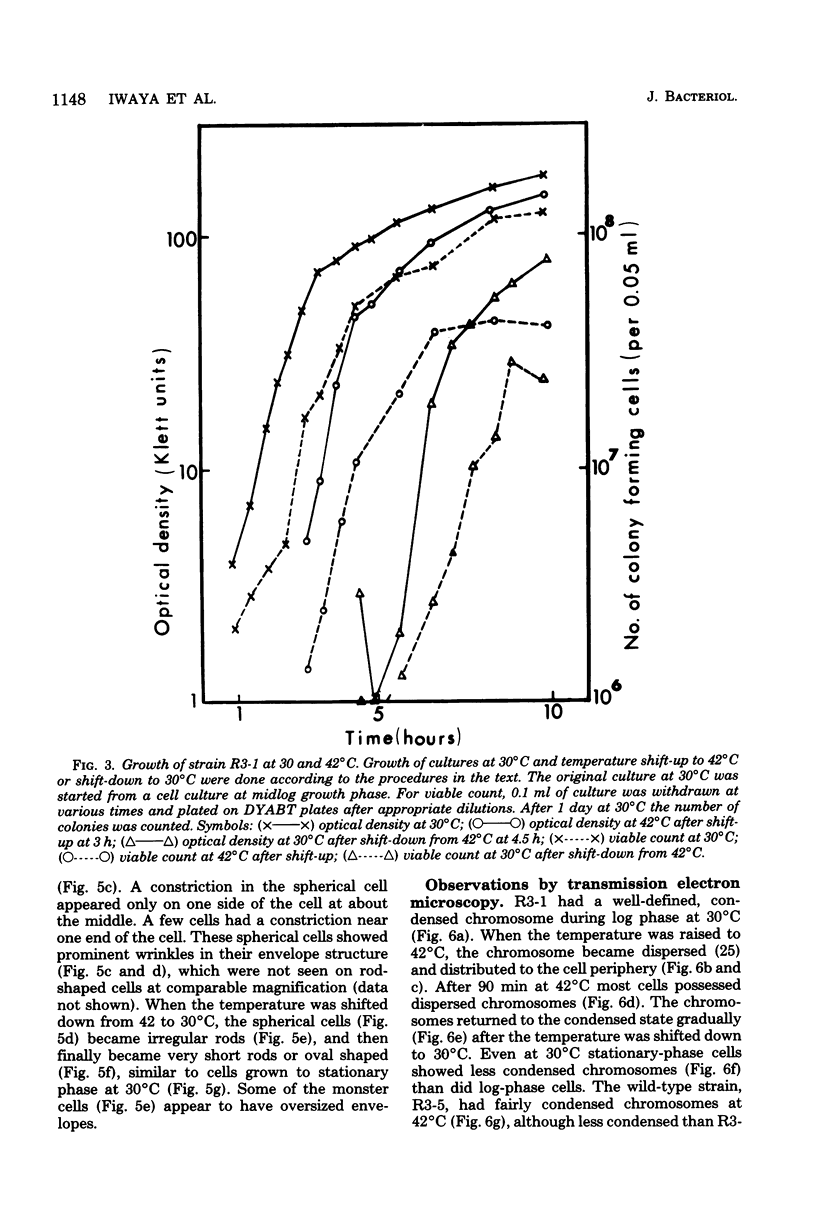
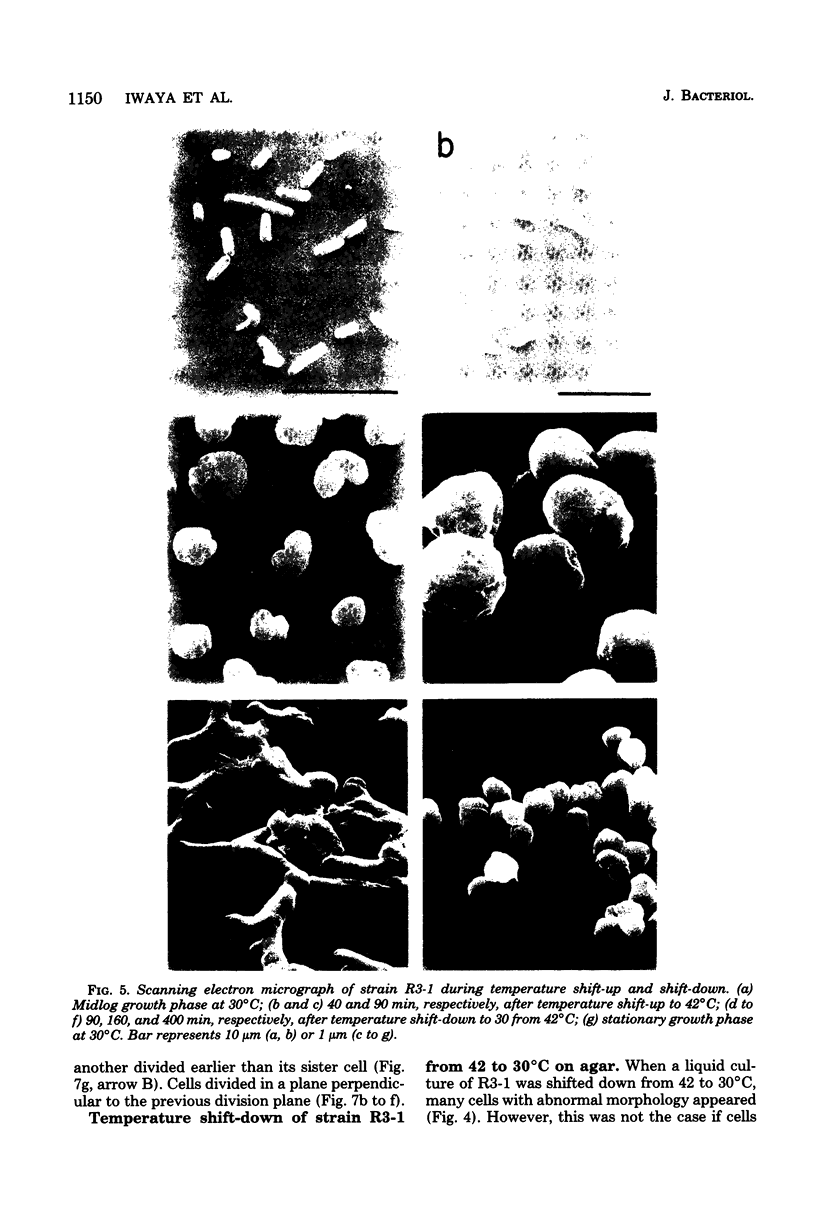
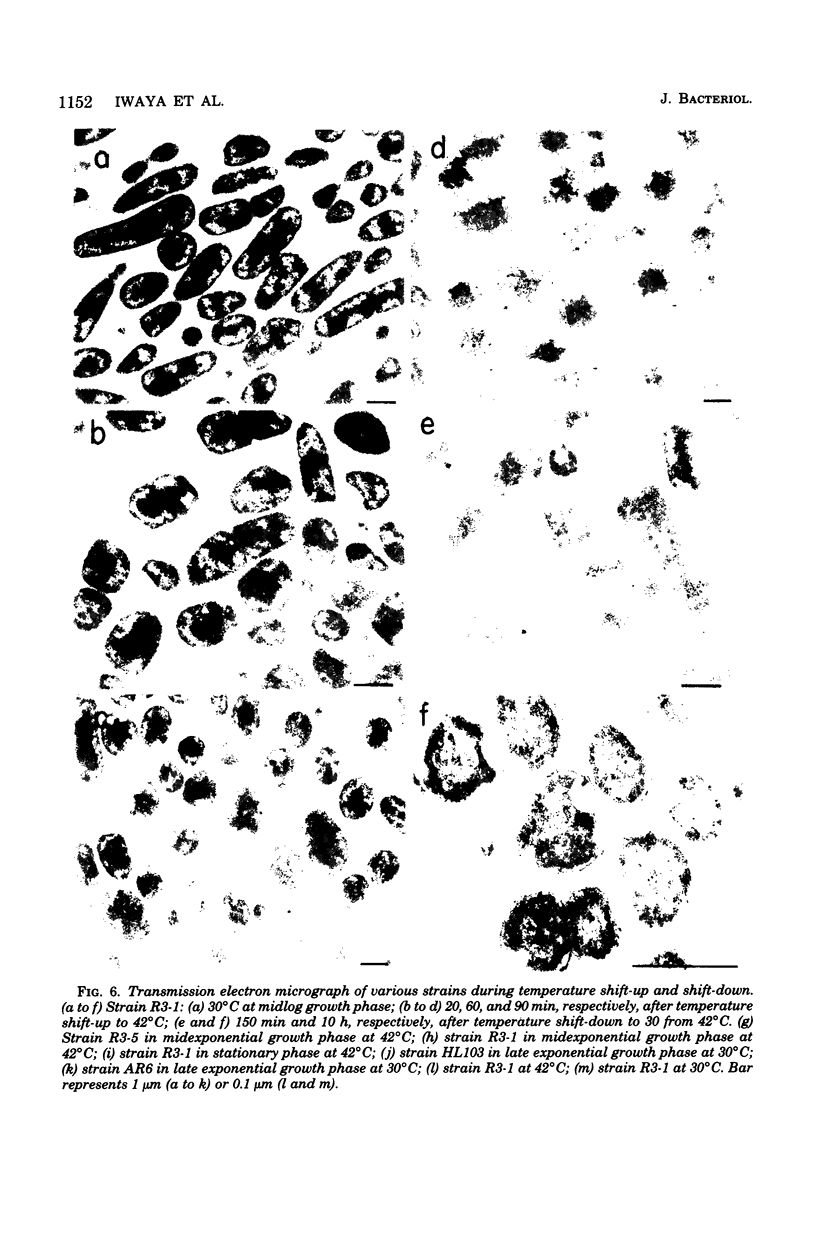
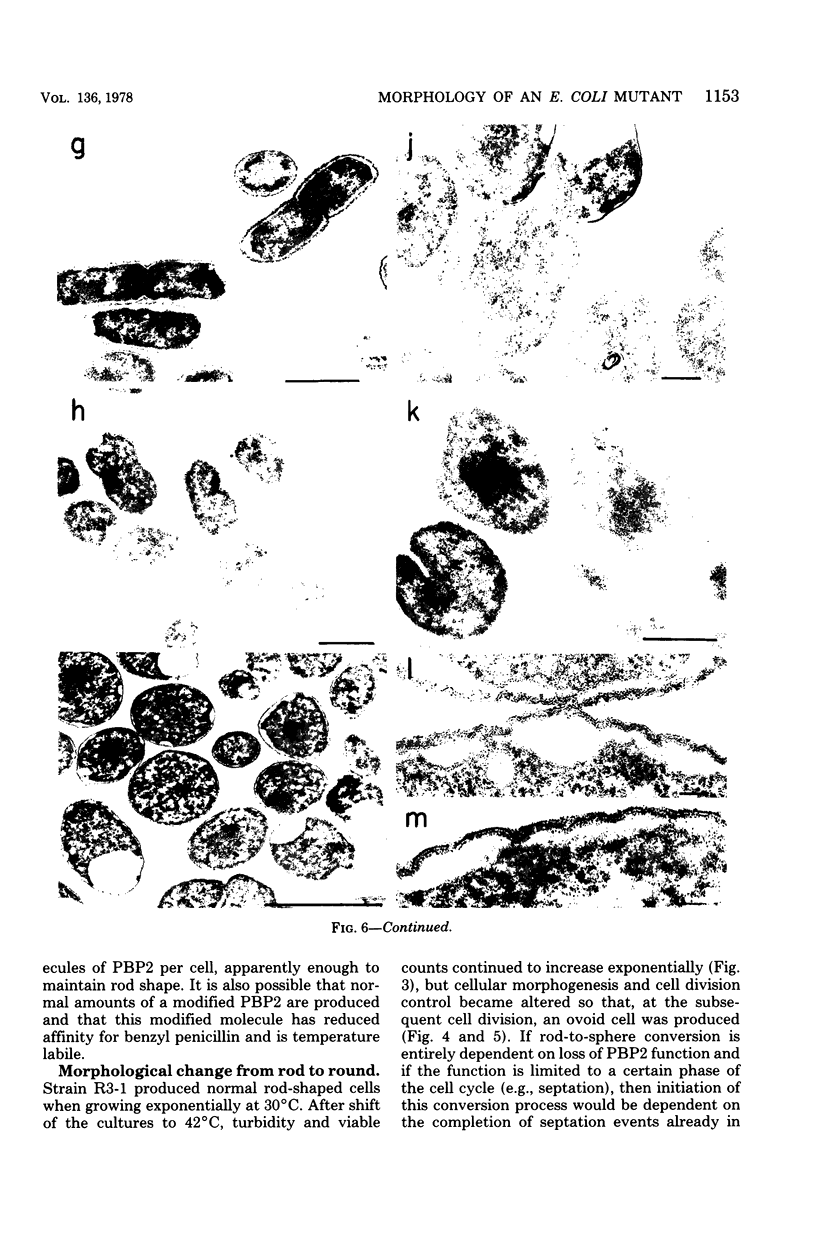
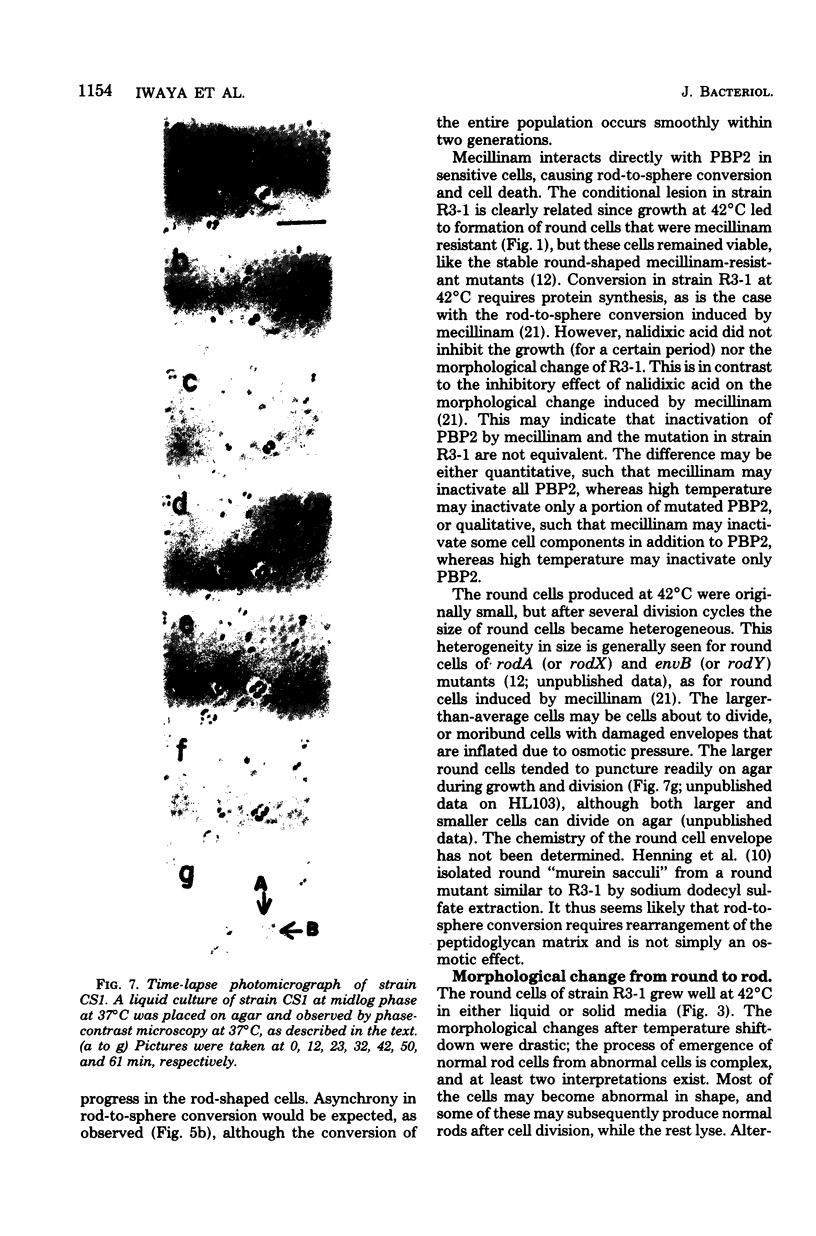
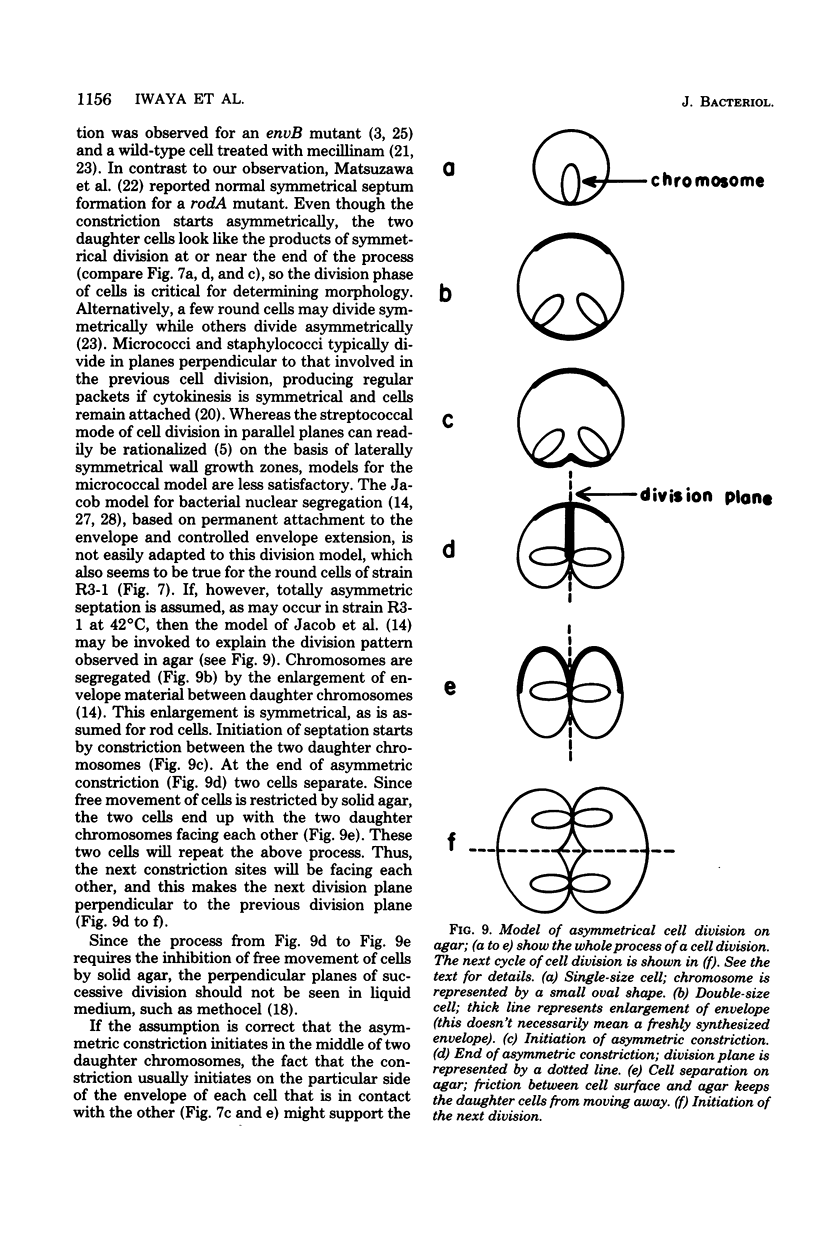
Mutants of Escherichia coli capable of growing in the presence of 10 microgram of mecillinam per ml were selected after intensive mutagenesis. Of these mutants, 1.4% formed normal, rod-shaped cells at 30 degrees C but grew as spherical cells at 42 degrees C. The phenotype of one of these rod(Ts) mutants was 88% cotransducible with lip (14.3 min), and all lip+ rod(Ts) transductants of a lip recipient had the following characteristics: (i) growth was relatively sensitive to mecillinam at 30 degrees C but relatively resistant to mecillinam at 42 degrees C; (ii) penicillin-binding protein 2 was present in membranes of cells grown at 30 degrees C in reduced amounts and was undetectable in the membranes of cells grown at 42 degrees C. The mecillinam resistance, penicillin-binding protein 2 defect, and rod phenotypes all cotransduced with lip with high frequency. Thus the mutation [rodA(Ts)] is most likely in the gene for penicillin-binding protein 2 and causes the organism to grow as a sphere at 42 degrees C, although it grows with normal rodlike morphology at 30 degrees C. At 42 degrees C, cells of this strain were round with many wrinkles on their surfaces, as revealed by scanning electron microscopy. In these round cells, chromosomes were dispersed or distributed peripherally, in contrast to normal rod-shaped cells which had centrally located, more condensed chromosomes. The round cells divided asymmetrically on solid agar, and it seemed that the plane of each successive division was perpendicular to the preceding one. On temperature shift-down in liquid medium many cells with abnormal morphology appeared before normal rod-shaped cells developed. Few abnormal cells were seen when cells were placed on solid medium during temperature shift-down. These pleiotropic effects are presumably caused by one or more mutations in the rodA gene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Terry C. E., Hardigree A. A. Giant cells of Escherichia coli. J Bacteriol. 1968 Jan;95(1):139–142. doi: 10.1128/jb.95.1.139-142.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison D. P. Giant cells of Escherichia coli: a morphological study. J Bacteriol. 1971 Dec;108(3):1390–1401. doi: 10.1128/jb.108.3.1390-1401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman D. A., Weinbaum G. The formation of multiple layers of membrane-like structures in Escherichia coli B. J Cell Biol. 1967 Feb;32(2):524–528. doi: 10.1083/jcb.32.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. FL 1060: a new beta-lactam antibiotic with novel properties. J Clin Pathol. 1973 Jan;26(1):1–6. doi: 10.1136/jcp.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Henning U., Haller I. Mutants of Escherichia coli K12 lacking all 'major' proteins of the outer cell envelope membrane. FEBS Lett. 1975 Jul 15;55(1):161–164. doi: 10.1016/0014-5793(75)80983-5. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Braun V., Höhn B. Cell envelope and shape of Escherichia coli K12. Properties of a temperature-sensitive rod mutant. Eur J Biochem. 1972 Apr 24;26(4):570–586. doi: 10.1111/j.1432-1033.1972.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Iwaya M., Jones C. W., Khorana J., Strominger J. L. Mapping of the mecillinam-resistant, round morphological mutants of Escherichia coli. J Bacteriol. 1978 Jan;133(1):196–202. doi: 10.1128/jb.133.1.196-202.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Properties of adenyl cyclase and cyclic adenosine 3',5'-monophosphate receptor protein-deficient mutants of Escherichia coli. J Bacteriol. 1976 Feb;125(2):545–555. doi: 10.1128/jb.125.2.545-555.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli. VI. Use of a methocel-autoradiographic method for the study of cellular division in Escherichia coli. J Bacteriol. 1971 Oct;108(1):375–385. doi: 10.1128/jb.108.1.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. M. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956 Apr;71(4):474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Hayakawa K., Sato T., Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior N. H., Blom J., Tybring L., Birch-Andersen A. Light and electron microscopy of the early response of Escherichia coli to a 6beta-amidinopenicillanic acid (FL 1060). Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Aug;81(4):393–407. doi: 10.1111/j.1699-0463.1973.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Bacitracin induces sphere formation in Halobacterium species which lack a wall peptidoglycan. J Gen Microbiol. 1975 Aug;89(2):375–378. doi: 10.1099/00221287-89-2-375. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Jacob F. DNA-membrane complex and nuclear segregation in bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:669–676. doi: 10.1101/sqb.1968.033.01.076. [DOI] [PubMed] [Google Scholar]
- Sanders S. K., Alexander E. L., Braylan R. C. A high-yield technique for preparing cells fixed in suspension for scanning electron microscopy. J Cell Biol. 1975 Nov;67(2PT1):476–480. doi: 10.1083/jcb.67.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. The mechanism of action of mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):13–19. doi: 10.1093/jac/3.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- Westling-Häggström B., Normark S. Genetic and physiological analysis of an envB spherelike mutant of Escherichia coli K-12 and characterization of its transductants. J Bacteriol. 1975 Jul;123(1):75–82. doi: 10.1128/jb.123.1.75-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L. Morphological analysis of nuclear separation and cell division during the life cycle of Escherichia coli. J Bacteriol. 1976 Jan;125(1):248–257. doi: 10.1128/jb.125.1.248-257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]