Abstract
The role of dynamin GTPases in the regulation of receptor-mediated endocytosis is well established. Here, we present new evidence that the ubiquitously expressed isoform dynamin-2 (dyn2) can also function in a signal transduction pathway(s). A ≤5-fold increase of dyn2 relative to endogenous levels activates the transcription factor p53 and induces apoptosis, as demonstrated by reduced cell proliferation, DNA fragmentation, and caspase-3 activation. Dyn2-triggered apoptosis occurs only in dividing cells and is p53 dependent. A mutant defective in GTP binding does not trigger apoptosis, indicating that increased levels of dyn2·GTP, rather than protein levels per se, are required to transduce signals that activate p53. A truncated dyn2 lacking the COOH-terminal proline/arginine-rich domain (PRD), which interacts with many SH3 domain-containing partners implicated in both endocytosis and signal transduction, triggers apoptosis even more potently than the wild-type. This observation provides additional support for the importance of the NH2-terminal GTPase domain for the apoptotic phenotype. All described effects are dyn2-specific because >200-fold overexpression of dyn1, the 70% identical neuronal isoform, has no effect. Our data suggest that dyn2 can act as a signal transducing GTPase affecting transcriptional regulation.
Keywords: dynamin, apoptosis, p53, GTPase, endocytosis
Introduction
Dynamin is a member of the GTPase superfamily whose role in endocytosis was first revealed by phenotypic analysis of temperature-sensitive mutations in the Drosophila homologue, shibire (reviewed in Warnock and Schmid 1996; Urrutia et al. 1997; Schmid et al. 1998). Dynamin's role in receptor-mediated endocytosis in mammalian cells has been confirmed both in vivo by overexpression of dominant-negative mutants of dynamin (Herskovits et al. 1993; van der Bliek et al. 1993; Damke et al. 1994) and in vitro (Simpson et al. 1999), but its exact function remains controversial (Sever et al. 2000). Some models suggest that dynamin functions as a mechanochemical enzyme to drive membrane fission (Hinshaw and Schmid 1995; Warnock and Schmid 1996; McNiven 1998; Smirnova et al. 1999; Stowell et al. 1999). However, recent results argue that dynamin functions like all other members of the GTPase superfamily, as a regulatory molecule to activate downstream effectors directly required for coated vesicle formation (Sever et al. 1999).
Dynamin is highly conserved in multicellular organisms throughout evolution: the Drosophila and C. elegans homologues of dynamin are 70 and 61% identical to human dynamin, respectively. While both Drosophila and C. elegans carry only a single dynamin gene, mammals express three dynamin isoforms in a tissue-specific manner. Each of these isoforms is ∼70% identical to each other and equally homologous to shibire (Urrutia et al. 1997; van der Bliek 1999). The majority of studies to date have focused on the neuron-specific isoform dynamin-1 (dyn1). Dynamin-2 (dyn2) is ubiquitously expressed and dynamin-3 (dyn3) is predominantly expressed in testes and, to a lesser extent, in neurons. In addition, there are numerous splice variants for each isoform which suggest that, at least in mammals, these diverse dynamin family members might participate in distinct roles other than receptor-mediated endocytosis (McNiven et al. 2000). Here, we report that dyn2 can function as a signaling GTPase as demonstrated by the induction of p53-dependent apoptosis in dividing cells.
Apoptosis (programmed cell death) is a highly regulated response to specific cellular signals and is distinct from necrosis in both the biochemical and the morphological changes that occur. In contrast to necrotic cells, apoptotic cells are characterized by shrinkage of the cytoplasm and production of membrane-bound apoptotic bodies. Biochemically, apoptosis is distinguished by fragmentation of the genome and activation of caspases that cleave several cellular proteins (Darzynkiewicz et al. 1997). Some, but not all apoptotic pathways are dependent on activation of the transcription activator and tumor suppressor, p53 (Levine 1997).
Levels of expression and activity of p53 are increased in response to a variety of cellular stresses including, but not limited to, genotoxic stress, oxidative stress, and oncogene activation (Choisy-Rossi et al. 1998; Ding and Fisher 1998; Evan and Littlewood 1998; Burns and El-Deiry 1999). Upon activation, p53 enters the nucleus and triggers a cascade of events that can lead to either cell cycle arrest or apoptosis depending on the cell type, its environment, its rate of cell division, and other poorly understood factors. Greater than 50% of human cancers carry mutations in p53 and given its central role in responding to cellular insults, it has been referred to as the cellular gatekeeper (Levine 1997) or guardian of the genome (Lane 1992).
Cellular levels of p53 are largely controlled posttranslationally by its rapid ubiquitin- and proteasome-dependent turnover (Blagosklonny 1997). p53 activity is also regulated by site-specific phosphorylation and nuclear translocation (reviewed in Burns and El-Deiry 1999). There has been considerable recent progress in identifying molecules and mechanisms of regulating p53 interaction with the ubiquitin-mediated proteolysis pathway (Lane and Hall 1997; Prives 1998), but few of the upstream signaling events impinging on these pathways have been identified. Our experimental data support the hypothesis that the GTPase dynamin-2 is a component of a tightly regulated signaling pathway with the potential to act as an upstream regulator of the transcription factor p53.
Materials and Methods
Cells, Antibodies, and Reagents
HeLa cells stably expressing the tetracycline-regulatable chimeric transcription activator (tTA-HeLa) were obtained from H. Bujard (Gossen and Bujard 1992) and cultured as previously described (Damke et al. 1994; Altschuler et al. 1998). The p53−/− and p53+/+ mouse embryo fibroblasts were kindly provided by Geoffrey Wahl (The Salk Institute); the p53-deficient and p53ts Burkitt Lymphoma tumor cell lines (Akata) were kindly provided by Weiping Chen (The Scripps Research Institute). Peripheral blood mononuclear cells (PBMC) were isolated from the blood of a pool of donors from the GCRC at Scripps Green Hospital and cultured as previously described (Fish et al. 1995). 5 × 107 PBMC were plated onto 60-mm Primaria culture dishes (Becton Dickinson) and incubated at 37°C in 7% CO2 for infections. Adherent cells were induced to differentiate by cocultivation with concanavalin A–treated nonadherent cells for 24 h (Ibanez et al. 1991). Subsequently, all nonadherent cells were removed and the remaining macrophages were cultured as previously described (Fish et al. 1995). The adherent cells were >99% esterase positive at 72 h (Ibanez et al. 1991).
The following antibodies were used in this study: anti-dynamin mouse monoclonal hudy-1 (Warnock et al. 1995) and the anti-pan dynamin rabbit polyclonal, 748 (van der Bliek et al. 1993), prepared in this laboratory; anti-hemagglutinin epitope mouse monoclonal, 12CA5 (Boehringer Mannheim); and anti-p53 mouse monoclonal antibody DO-1 (Santa Cruz Biotechnology, Inc.).
Adenovirus Production and Infection
Recombinant adenoviruses encoding HA-dyn1wt (human dynamin-1aa splice variant, Ad-Dyn1wt), HA-dyn2wt (rat dynamin-2ba splice variant, Ad-Dyn2wt), HA-dyn2K44A (Ad-Dyn2K44A), and HA-dyn2ΔPRD (Ad-Dyn2ΔPRD) under control of a tetracycline-regulatable promoter were prepared as previously described (Altschuler et al. 1998). These adenoviral vectors are gutless, lacking most of the viral genes (Hardy et al. 1997). Recombinant adenoviruses expressing the tetracycline regulatable transactivator (tTA) (Ad-tTA, kindly provided by Dr. Yoram Altschuler, University of California, San Francisco) were used as a control virus and for driving dynamin expression in cells lacking tTA as described in Streblow et al. 1999. For infection, tTA-HeLa cells were incubated for 2 h with an moi of ∼10 pfu/cell (unless otherwise indicated) in Hank's buffered saline solution as described (Altschuler et al. 1998). Cells were then washed twice with tetracycline-free media and incubated for the indicated times before experimental analysis. Under these conditions, >90% of the cells were positive for HA expression by 24 h postinfection as determined by indirect immunofluorescence. For all experiments, conditions with similar frequency of infected cells between Ad-Dyn1– and Ad-Dyn2–infected cultures were chosen.
Flow Cytometry
Subconfluent tTA-HeLa cultures in 10-cm dishes were infected with Ad-Dyn1, Ad-Dyn2, Ad-Dyn2K44A, or Ad-tTA as described above. Uninfected and infected cell cultures were rinsed, nonadherent cells were saved, and adherent cells were removed from the dish by exposure to trypsin. Trypsinized cells were combined with non-adherent cells, rinsed once in medium containing serum and then resuspended at a concentration of 107 cells/ml in PBS. Cells were then fixed by adding 0.5 ml of cell suspension to 4.5 ml 70% ice-cold ethanol. After 2 h on ice, cells were pelleted and the ethanol was thoroughly decanted. The cell pellet was rinsed in 5 ml PBS, centrifuged, resuspended in 1 ml of PBS containing 0.1% Triton X-100, 0.2 mg/ml DNase-free RNase A, and 20 μg/ml propidium iodide (PI), and incubated at 37°C for 15 min before FACS® analysis. Cells were analyzed using Becton Dickinson Cell Quest FACStation software (version 3.0.1) operating a Becton Dickinson FACSCalibur FACS machine. Gating was used to remove debris and doublets before collection. Results were quantitated using ModFit LT (1999; Topsham, Maine: Verity Software House, Inc.).
Assays for Apoptosis
Early events in apoptosis leading to loss of plasma membrane integrity were detected using the fluorescent DNA dye, YO-PRO-1® (Molecular Probes) at 1 μM, according to the manufacturer's instructions. Dead cells were detected with propidium iodide staining at 5 μg/ml. Mitochondrial membrane potential was assessed by incubating cell cultures with 25 nM MitoTracker® Red CMXRos (Molecular Probes) according to manufacturer's instructions. Cell viability was assessed by Trypan blue staining or by an MTT ([(3-4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] conversion assay (Mosmann 1983) performed as previously described (Behl et al. 1994).
To assess caspase-3 activation, infected cells were harvested at different times postinfection and resuspended at a final concentration of 2 × 106 viable (Trypan blue negative) cells/ml in 10 mM Tris-HCl (pH 7.5), 10 mM NaH2PO4 (pH 7.5), 130 mM NaCl, 1% Triton-X 100, 10 mM Na pyrophosphate, and placed on ice for 30 min. For each caspase-3 activity assay 5 μl of 1 μg/ml Ac-DEVD-AMC (PharMingen) was combined with 500 μl of 20 mM Hepes (pH 7.5), 10% glycerol, 4 mM DTT, and 100 μl of cell suspension. Reaction mixtures were then incubated for 30 min at 37°C and diluted 50% with ddH2O before the fluorometric quantification of released AMC using excitation at 380 and 435 nm as emission wavelength.
Immunocytochemistry
For immunofluorescence, cells were grown and infected on chamber slides. Cells were fixed for 20 min at room temperature in 4% paraformaldehyde and permeabilized with 0.3% Triton X-100 in PBS. Samples were preblocked with 20% normal goat serum in PBS and incubated for 1 h at 37°C with the indicated primary antibodies. Binding of primary antibody was detected using the appropriate Alexa-488 conjugated goat anti–mouse (Molecular Probes). The Slowfade antifade kit (Molecular Probes) was used to reduce fluorescence fading. Cells were visualized using a DeltaVision Optical Sectioning Microscope Model 283. This system consists of an Olympus IX-70 microscope equipped with a mercury arc lamp, a Photometrics CH 350 cooled CCD camera, and a high precision motorized XYZ stage, which was used to acquire multiple sections at a 0.2-μm interval for each of the fluorescent probes. After data acquisition, the data was deconvolved using DeltaVision software version 2.1 based on the Agard/Sadat inverse matrix algorithm and recompiled so as to be representative of total cellular fluorescence.
Online Supplemental Material
Figure S1 depicts how dynamin-2 expression triggers DNA fragmentation without cell cycle arrest, whereas Figure S2 shows dynamin-2–induced apoptosis detected by disruption of mitochondrial membrane potential. Supplemental figures and their respective legends are available at http://www.jcb.org/cgi/content/full/150/1/145/DC1.
Results
Functional characterization of dynamin has been greatly facilitated by the use of stable HeLa cell lines overexpressing wild-type and dominant-negative mutants of dyn1 under control of a tetracycline regulatable promoter (Damke et al. 1994, Damke et al. 1995). Our repeated attempts to generate similar stable HeLa cell lines inducibly expressing dynamin-2 were unsuccessful. Although in this procedure cells are transfected and selected in the presence of tetracycline to tightly control the expression levels of potentially toxic proteins, there is always some leakiness, especially at early times after transfection. These results suggested that even transient overexpression of low levels of dyn2 was extremely toxic to HeLa cells.
To circumvent this problem and explore the cellular function of dyn2, we generated recombinant adenoviruses encoding wt and mutant dyn1 and dyn2, designated Ad-Dyn1 and Ad-Dyn2, under control of a tetracycline-regulatable promoter (Altschuler et al. 1998). The use of a tet-regulatable promoter enables efficient large-scale production of viruses that encode toxic proteins. At a multiplicity of infection (moi) of 10 pfu/cell, adenovirus infection of tTA-HeLa cells expressing the tet-responsive, chimeric transcription activator tTA results in uniform levels of dynamin expression in >90% of cells (data not shown). Therefore, unless otherwise indicated, this moi was used. Importantly, expression levels of the transferred dynamin genes can be controlled either by altering the moi or the amount of tetracycline in the medium. These adenoviruses have recently been used to show that, when assayed 12–18 h after infection, receptor-mediated endocytosis is selectively inhibited by overexpression of either dyn1K44A or dyn2K44A (Altschuler et al. 1998), whereas overexpression of wild-type dyn1 or dyn2 had no effect (Altschuler et al. 1998). Thus, these comparative findings failed to provide an explanation, with regards to membrane trafficking, for the presumed toxic effects of increased dyn2 expression on cells.
Increased GTP-bound Dynamin-2 Is Cytotoxic
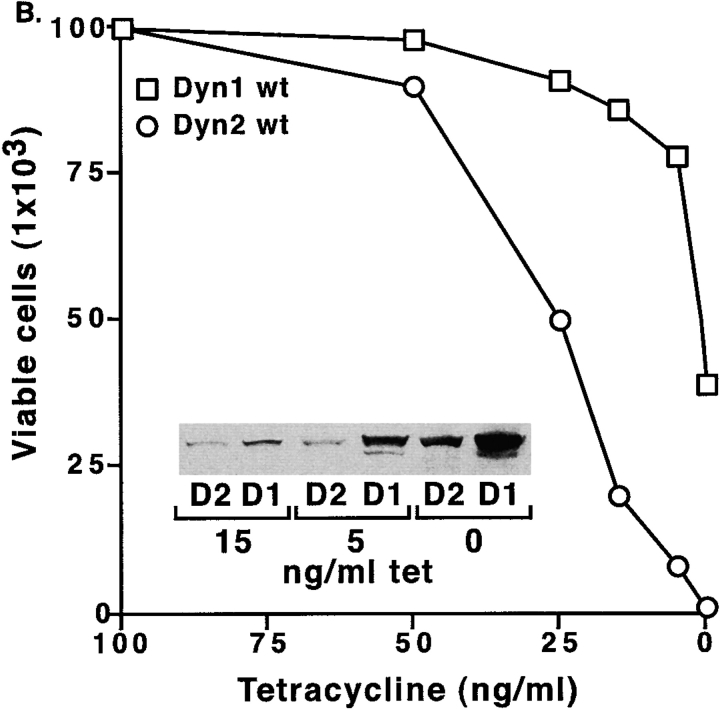
When tTA-HeLa cells were infected with these viruses and observed at 24–36 h postinfection, there were clear signs of cytopathology in the cultures overexpressing dyn2wt when compared with cultures expressing either dyn1wt or dyn2K44A (Fig. 1). Importantly, we used a gutless adenoviral vector (Hardy et al. 1997) that lacks viral genes known to be cytotoxic (McPake et al. 1999), and controlled all experiments for nonspecific, virus-induced effects by infection with adenovirus expressing the transactivator protein, tTA (Ad-tTA). Moreover, the cytotoxicity observed at 36 h postinfection (Fig. 1 A) with Ad-Dyn2wt was specifically dependent on levels of dyn2wt expression since it was reduced when cells were cultured in the presence of low amounts of tetracycline and blocked at concentrations of tetracycline that suppress protein expression (Fig. 1 B). At high levels of overexpression of dyn2K44A, some cell rounding could be observed (Fig. 1 A); however dyn2K44A was considerably less toxic than dyn2wt (data not shown and see Fig. 4). Under these conditions, there were no discernible cytotoxic effects in cells infected with either Ad-Dyn1wt (Fig. 1) or Ad-Dyn1K44A (data not shown). The K44A mutation in dynamin results in decreased affinity for guanine nucleotides (Damke et al. 1994; Warnock et al. 1995), thus the differential effects of dyn2wt and dyn2K44A suggest, as is the case for oncogenic GTPases, that toxicity is due to increased levels of GTP-bound dyn2 rather than of the protein per se.
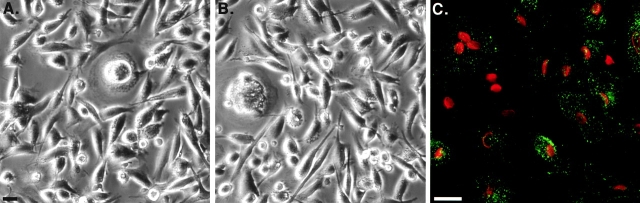
Figure 1.
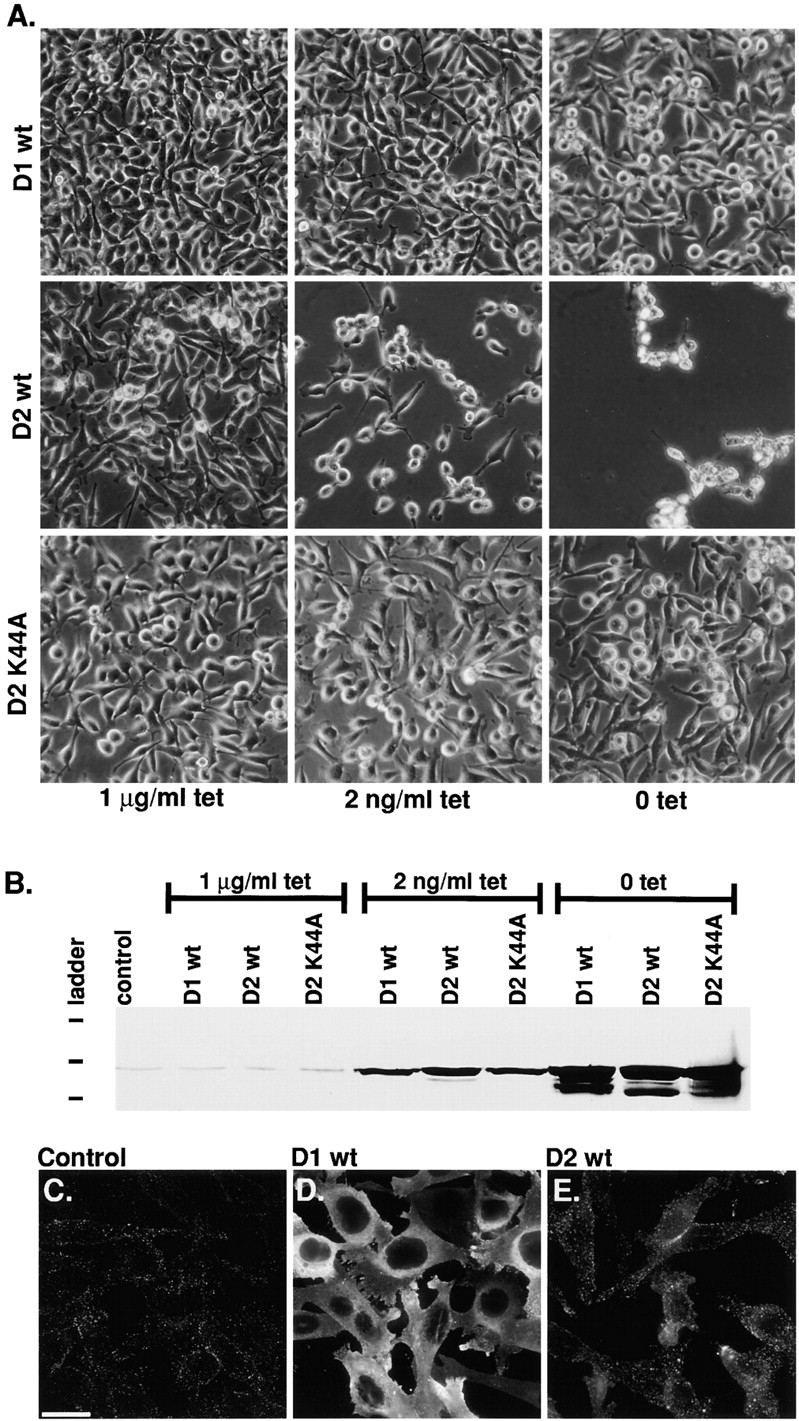
Dynamin-2 specifically induces cell toxicity in tTA HeLa cells. (A) Phase contrast micrographs of tTA-HeLa cells infected with an equivalent moi of adenovirus expressing either dyn1wt, dyn2wt, or dyn2K44A under control of a tetracycline-regulatable promoter, as described in Materials and Methods. After infection, cells were incubated for 36 h in media containing the indicated concentration of tetracycline. (B) Western blot analysis using the pan-dynamin antibody 748 showing endogenous dynamin-2 (detected in control lanes and in lanes where exogenous expression is suppressed in the presence of 1 μg/ml tet) and tetracycline-dependent expression levels of exogenous dyn1wt, dyn2wt, or dyn2K44A. (C–E) Immunofluorescence images of dynamin staining in uninfected tTA HeLa cells (C) or tTA HeLa cells infected with adenovirus encoding dyn1wt (D) or dyn2wt (E) and cultured for 24 h in the absence of tetracycline. Bar, 20 μm.
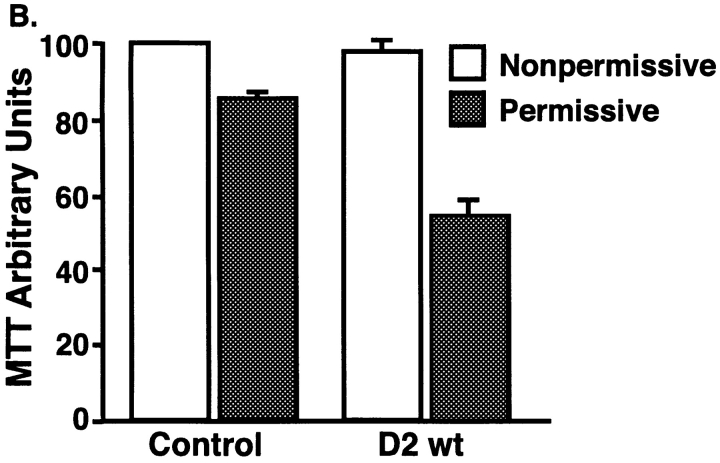
Figure 4.
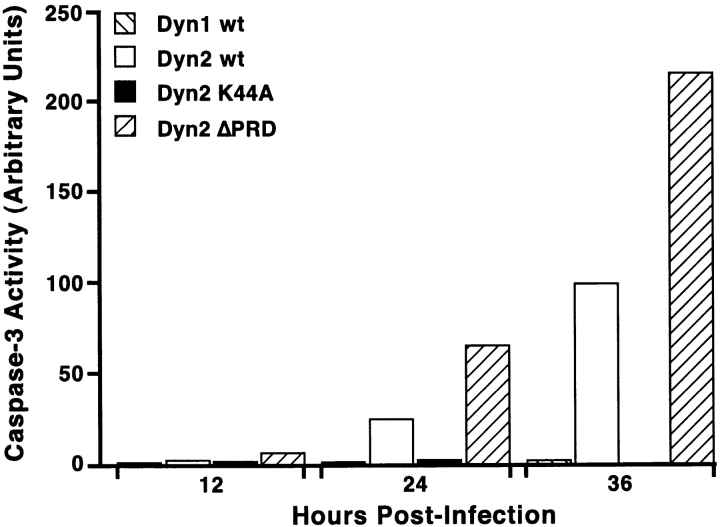
Dynamin-2–induced apoptosis detected by increased caspase-3 activity. tTA-HeLa cells infected with adenovirus encoding dyn1wt or dyn2wt or the dynamin mutants dyn2K44A and dyn2ΔPRD. Cells were harvested at the indicated times postinfection and viable cells were assayed for caspase-3 activity using the fluorescent substrate Ac-DEVD-AMC as described in Materials and Methods. For comparison of independent experiments, caspase-3 activity is plotted in arbitrary units setting dyn2wt at 36 h postinfection as 100 after substracting the background of tTA-infected cells. Representative data of three independent experiments are shown.
At the low levels of expression sufficient to cause toxicity, exogenous dyn2wt is localized to punctate structures at the cell surface similar to endogenous dynamin (compare Fig. 1C and Fig. E). Dyn1 does not cause cytotoxicity even when expressed at high concentrations that saturate the membrane binding sites (Damke et al. 1994), resulting in cytosolic accumulation (Fig. 1 D). The selective cytotoxicity of dyn2wt is not due to its effects on endocytosis, because overexpression of wild-type dyn2 does not inhibit endocytosis (Altschuler et al. 1998; Kasai et al. 1999). Moreover, overexpression of dyn2K44A, which inhibits endocytosis, is considerably less toxic.
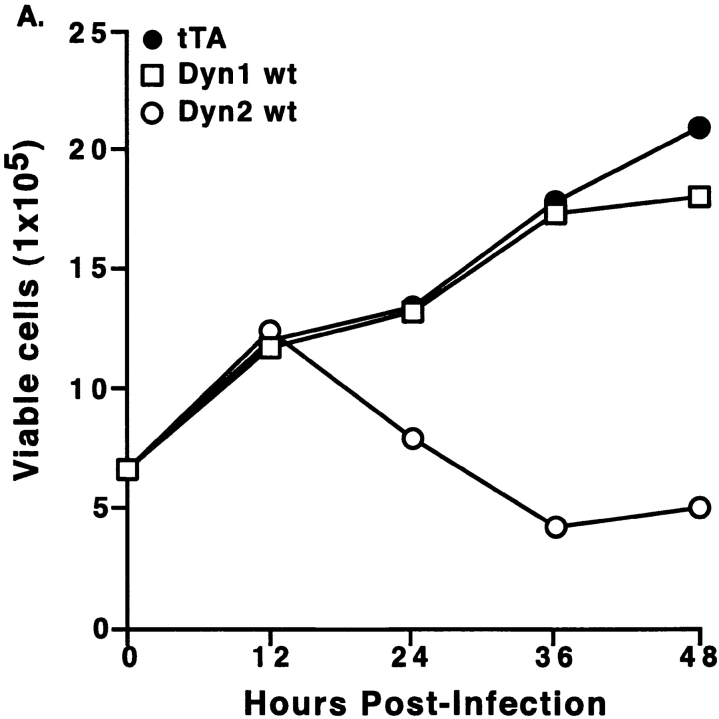
The specific effects of overexpression of dyn2wt were quantitated using Trypan blue exclusion as a measure of cell viability. Infection of cells with Ad-Dyn2wt resulted in loss of viability starting 24 h after infection (Fig. 2 A). Similar results were obtained using MTT conversion assays (data not shown). Reduced cell proliferation was detected as early as 16 h postinfection, but was most striking after 36 h. Infection with equivalent amounts of Ad-Dyn1wt or Ad-tTA had no effect (Fig. 2 A). However, when cells were infected with a very high moi of 500, Ad-Dyn1wt was found to be toxic, albeit still at considerably reduced levels compared with Ad-Dyn2wt (Fig. 2 B). Even at this high moi, no toxicity was observed with Ad-tTA (not shown). By varying the concentration of tetracycline in the medium we could regulate the levels of expression of dyn1wt and dyn2wt to quantitate the differences in toxicity (Fig. 2 B). We found that as little as a fivefold increase in dyn2wt expression resulted in a substantial decrease in cell viability. Quantitation of dynamin expression by Western blotting (Fig. 2 B, inset) suggested that dyn2wt was at least 50-fold more toxic than its closely related isoform, dyn1wt.
Figure 2.
At low levels of expression, dynamin-2 specifically induces cell death. (A) Equal numbers of tTA-HeLa cells were infected with 10 moi of adenovirus expressing either dyn1wt(□), dyn2wt (○), or tTA (•). Cells were harvested at the indicated times postinfection and analyzed using Trypan blue exclusion to quantify cell viability. (B) Equal numbers of tTA-HeLa cells were infected with a high moi (500) which was chosen to detect some toxicity of adenoviruses encoding dyn1wt, and incubated in the presence of varying concentrations of tetracycline to regulate expression of dyn1wt (□) and dyn2wt (○). Cells were harvested 36 h postinfection and analyzed for viability by Trypan blue exclusion. The insert in B shows Western blot analysis of corresponding levels of dyn1 and dyn2 expression under these conditions detected using the pan-dynamin polyclonal antibody 748. Dyn1wt at an moi 500 in the absence of tetracycline is expressed ∼100-fold over endogenous dyn2 as quantified by densitometric measurements of Western blots.
Dynamin-2 Specifically Induces an Apoptotic Pathway
Before dyn2-induced cell death, we observed a decrease in the rate of cell proliferation (Fig. 2 A), which may reflect a dyn2-dependent block in the cell cycle. To test this hypothesis, FACS® analysis was performed on uninfected tTA-HeLa cells or cells infected with Ad-Dyn1wt or Ad-Dyn2wt. Infected cultures were harvested at 18, 24, 28, 32, and 36 h postinfection. FACS® analysis was performed after gating to remove cell doublets. Although there were no visible changes in cell cycle distribution for any of the samples analyzed, an additional peak corresponding to the appearance of DNA fragmentation was, however, detected in the dyn2wt-expressing cells beginning at 24 h postinfection and increasing over time (data not shown and online material Fig. 1).
Apoptosis is a specific and highly regulated pathway of programmed cell death that can be distinguished from necrosis by a number of criteria (Darzynkiewicz et al. 1997). However, as no single parameter fully defines apoptosis, to establish the cell death pathway triggered by dyn2wt, we examined several criteria that distinguish live cells from early and late apoptotic cells and from necrotic cells. The methods we chose focused on chromatin structure, loss of plasma membrane integrity, changes in mitochondrial activity, and measurement of caspase-3 activity.
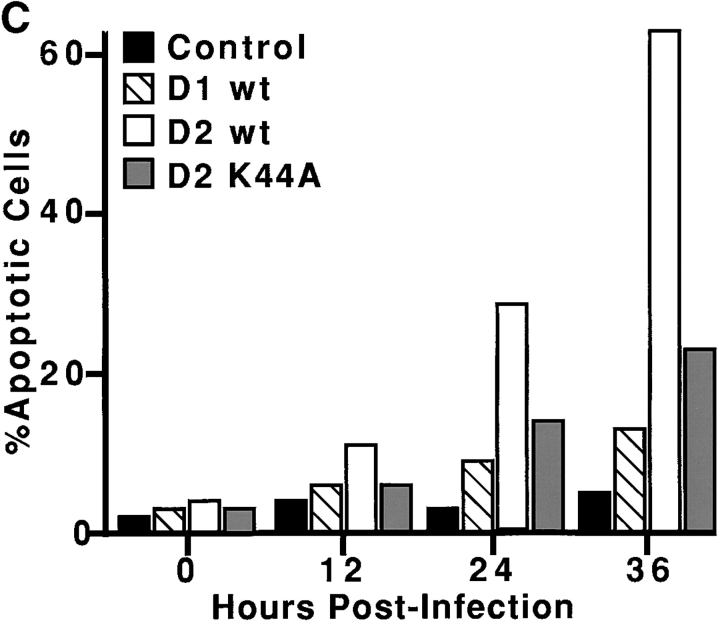
The characteristic breakdown of the nucleus during apoptosis comprises collapse and fragmentation of the chromatin, degradation of the nuclear envelope and nuclear blebbing, resulting in the formation of micronuclei. Therefore, nucleic acid stains can identify even low numbers of apoptotic cells in culture by fluorescence imaging. Thus, we used YO-PRO-1® (green fluorescence), which is taken up by cells during the initial stages of apoptosis before the breach in plasma membrane integrity, to identify early apoptotic cells (Idziorek et al. 1995). Propidium iodide (PI), a membrane impermeant dye (red fluorescence) was used to identify later stages of apoptosis and dead cells. The staining pattern resulting from the simultaneous use of these dyes in combination with Hoechst, a cell permeant DNA dye (blue fluorescence), makes it possible to distinguish normal (Hoechst positive/YO-PRO-1 negative/PI negative), early apoptotic (Hoechst + /YO-PRO-1 +/PI –) and dead (Hoechst +/YO-PRO-1 +/PI +) cells by fluorescence microscopy. All assays were performed on subconfluent tTA-HeLa cells that were uninfected or infected with Ad-Dyn1wt, Ad-Dyn2wt or Ad-Dyn2K44A. At different times postinfection YO-PRO-1, PI, and Hoechst were added to the cultures and incubated at 37°C for 10 min. Representative fields from Ad-Dyn1wt– and Ad-Dyn2wt–infected cells are shown in Fig. 3A and Fig. B, respectively, and the results are quantitated for all conditions (Fig. 3 C) as percent of viable (i.e., PI negative) cells. The results show that while there was some increase in apoptotic cells infected with Ad-Dyn1wt and Ad-Dyn2K44A over the uninfected control cells (shaded and hatched bars vs. black, respectively), a significantly higher percentage of cells infected with Ad-Dyn2wt became apoptotic between 12 and 24 h postinfection.
Figure 3.
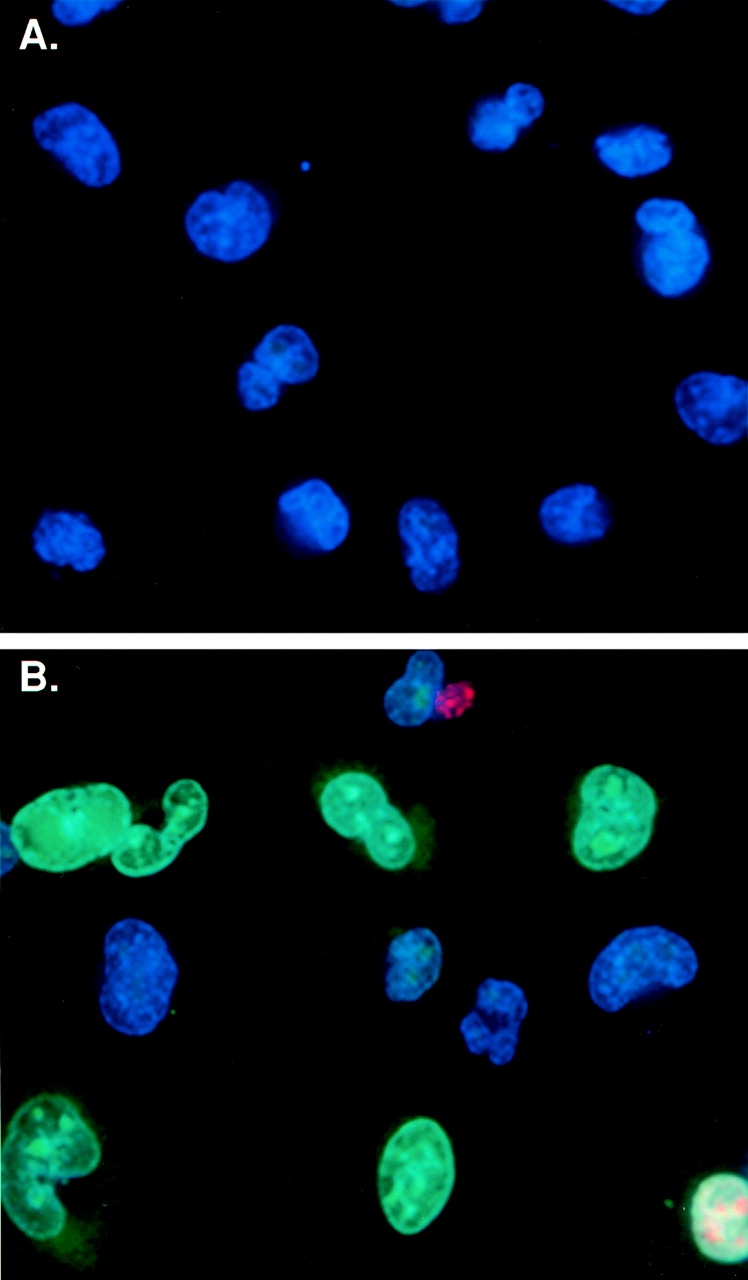
Dynamin-2–induced apoptosis detected by loss of membrane integrity. Subconfluent monolayers of tTA-HeLa cells were either uninfected or infected with adenovirus encoding either dyn1wt, dyn2wt, or dyn2K44A and incubated 24 h postinfection with the fluorescent DNA dyes, YO-PRO-1 (1 μM), propidium iodine (PI, 5 μg/ml) and Hoechst 33342 (10 μg/ml) for 10 min at 37°C as described in Materials and Methods. The immunofluorescence images shown are representative fields of adenovirally infected tTA-HeLa cells expressing dyn1wt (A) or dyn2wt (B) taken 24 h postinfection. Early apoptotic cells within the population with compromised plasma membrane integrity are labeled with YO-PRO-1® (green fluorescence). Cells at later stages of apoptosis and dead cells are labeled with the membrane impermeant dye, PI (red fluorescence). Hoechst, a cell permeant DNA dye (blue fluorescence), labels all cells in the population. Quantification of the number of early apoptotic cells (i.e., YO-PRO-1 positive), expressed as the percentage of viable cells (i.e., PI negative), at various times postinfection is shown in C. 100 cells were counted for each condition.
Another distinctive feature of apoptosis is the disruption of active mitochondria leading to the loss of membrane potential and alterations to the loss of the oxidation-reduction potential of the mitochondria (Green and Reed 1998). Indeed, infection of cells with Ad-Dyn2wt, but not Ad-Dyn1wt or control virus, resulted in loss of mitochondrial membrane potential as detected by the absence of stain using the fluorescent vital dye, MitoTracker Red CMXRos (Figure S2; Poot et al. 1996). DNA fragmentation was also visible at this time point (36 h postinfection) by Hoechst staining (Figure S2).
Finally, we examined the activity of caspase-3, a so-called effector caspase and a member of the caspase (CED-3/ICE) family of proteases that are crucial mediators of the complex biochemical events associated with apoptosis (Budihardjo et al. 1999; Thornberry and Lazebnik 1998). When activated, caspase-3 cleaves a number of different proteins at the sequence Asp-Glu-Val-Asp-⇓-X (DEVD-X) to trigger cellular changes associated with apoptosis. The fluorogenic tetrapeptide substrate Ac-DEVD-AMC was used to quantify caspase-3 activity of the different virally infected cultures. For these experiments, lysates from tTA-HeLa cells infected with adenovirus expressing wt and mutant forms of dyn1 and dyn2, were collected at various times postinfection to determine changes in caspase-3 activity (Fig. 4). The results are expressed in arbitrary fluorescent units for caspase activity over the background obtained from cells infected with control Ad-tTA virus. The values obtained for cells expressing Ad-Dyn2wt at 36 h postinfection were set at 100 to allow comparison between experiments. Caspase-3 activation was readily detected over background levels within 24 h in cells expressing dyn2wt, whereas no detectable activation was seen in cells expressing dyn1wt (compare open bars with backward slash).
We next explored the structural requirements for dyn2-induced caspase-3 activation. Strikingly, overexpression of dyn2K44A (shaded bars) also failed to induce caspase-3 activation as compared with control cells. This suggests that the slight toxicity observed in cells infected with Ad-Dyn2K44A was not due to apoptosis (Fig. 4). The greatest sequence divergence between dyn1 and dyn2 occurs in the COOH-terminal proline/arginine-rich domain (PRD). Moreover, this domain is known to interact with a number of SH3-domain containing signaling molecules. Therefore, we examined the ability of a truncated mutant of dyn2 lacking the PRD (dyn2ΔPRD) to induce caspase-3 activation. Surprisingly, infection of cells with Ad-Dyn2ΔPRD (forward slashed bars) induces a twofold increase of caspase-3 activity over dyn2wt. Inducible overexpression of dyn1ΔPRD in stably transformed tTA-HeLa cells does not induce apoptosis (Damke, H., and S.L. Schmid, unpublished results).
Together, these results clearly demonstrate that dyn2wt can selectively trigger apoptosis in HeLa cells. Importantly, efficient induction of apoptosis by dynamin-2 requires its ability to bind GTP but does not require the COOH-terminal PRD.
Dynamin-2–triggered Apoptosis Requires Cell Division
Low levels of dynamin-2 overexpression induced apoptosis in a variety of cell types studied, including U373 microglial cells, rat-1 fibroblasts, and primary fibroblasts (data not shown). However, induction of apoptosis or other cytotoxicity was not observed in earlier studies using contact-inhibited MDCK cell monolayers infected with Ad-Dyn2 (Altschuler et al. 1998). Similarly, when confluent, contact-inhibited monolayers of tTA HeLa cells were infected with the different adenoviruses, there was no discernible cytotoxicity; even though we could detect high levels of exogenous dynamin expression in these cultures (data not shown). These data suggested that while dyn2 expression had no detectable effect on the cell cycle (Figure S1), dyn2-induced apoptosis was, nonetheless, dependent on the cells' progression through the cell cycle. To further investigate these observations, we coinfected terminally differentiated, primary monocyte-derived macrophages with Ad-tTA and with either Ad-Dyn1wt (Fig. 5 A) or Ad-Dyn2wt (Fig. 5 B). Neither dynamin isoform induced cytotoxicity or apoptosis in these nondividing macrophages even though they were efficiently expressed in 60% of cells (Fig. 5 C). This cell cycle dependence strongly argues that the cytotoxic effect of dyn2wt overexpression is specific and highly regulated.
Figure 5.
Nondividing cells are resistant to dynamin-2–induced apoptosis. At 8 d post differentiation, primary monocyte-derived macrophage cultures were coinfected with 100 moi each of Ad-Trans and either Ad-Dyn1wt (A) or Ad-Dyn2wt (B) recombinant adenoviruses. These infection conditions were required to obtain maximum efficiency of ∼60% of cells expressing HA-dynamin as detected immunofluorescence using 12CA5 (C). Bars, 20 μm.
Dynamin-2–induced Apoptosis Is p53 Dependent
The data thus far establish that modest increases in dynamin-2 expression, resulting in increased levels of dyn2· GTP, can trigger cell cycle–dependent apoptosis. The cell cycle dependence coupled to the relatively slow and apparently asynchronous onset suggests that the apoptotic pathway triggered by dyn2·GTP might require p53. Indeed, immunofluorescence analysis revealed that p53 was specifically redistributed to the cell nuclei in dyn2wt-expressing cells as compared with tTA or dyn1wt-expressing cells (Fig. 6A and Fig. B, and data not shown). This redistribution was not due to an increase in p53 levels, which were similar in infected cells expressing tTA, dyn1wt or dyn2wt (as quantitated by Western analysis, data not shown). These results suggest that dyn2wt expression activates p53 as a regulator of transcription.
Figure 6.
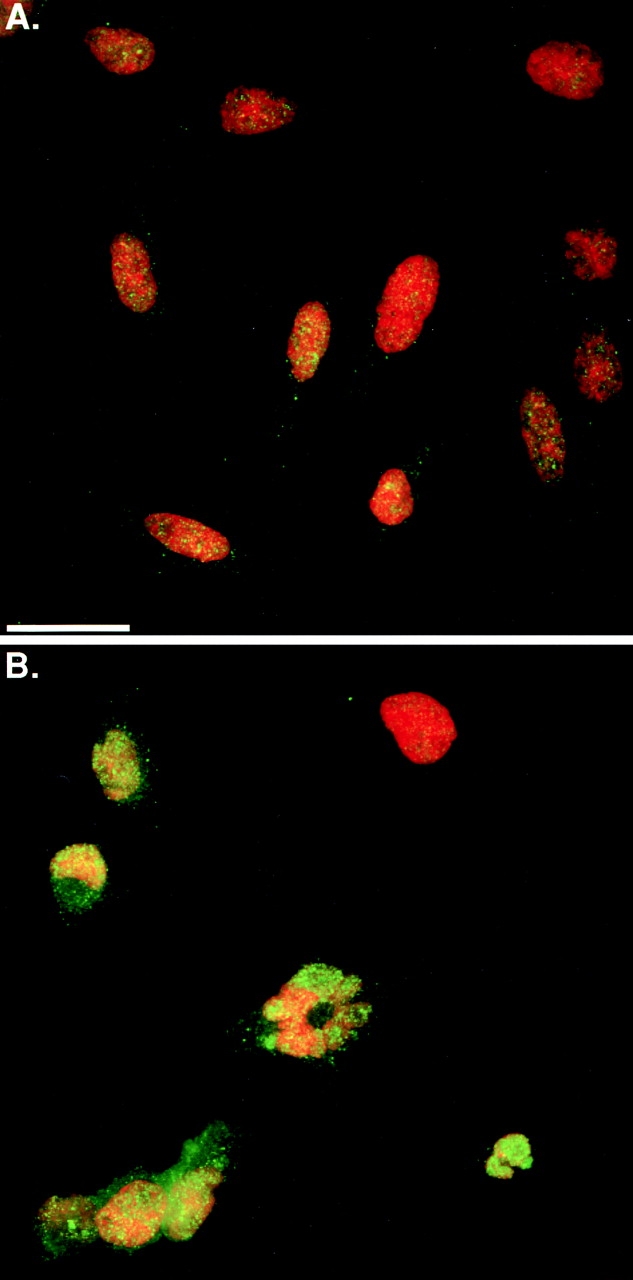
Dynamin-2 increases nuclear staining for p53. 24 h after adenoviral infection tTA Hela cells expressing dyn1wt (A) or dyn2wt (B) were processed for immunostaining with p53-specific antibodies (green). Nuclei (red) were stained with Hoechst. p53 redistribution to the nucleus was observed in >90% of dynamin-2–expressing cells. Bar, 20 μm.
To more directly establish a requirement for p53 in dyn2-triggered apoptosis, we examined the effect of dyn2 expression using p53-deficient mouse embryo fibroblasts (Jones et al. 1996). When coinfected with Ad-tTA and Ad-Dyn2wt p53−/− mouse fibroblasts (open bars) showed no discernible apoptosis as assessed by YO-PRO-1 staining. In contrast, 60% of p53+/+ cells (shaded bars) expressing dyn2wt were apoptotic within 24 h after infection (Fig. 7 A). Consistent with previous results (Fig. 3), expression of dyn2K44A also resulted in some YO-PRO-1–positive cells albeit at reduced levels as compared with dyn2wt. Importantly, the dyn2K44A-induced toxicity was not dependent on p53 as it was the same in both p53+/+ and p53−/− cells. Western blot analysis and immunofluorescence confirmed comparable dynamin expression in both cell lines (data not shown).
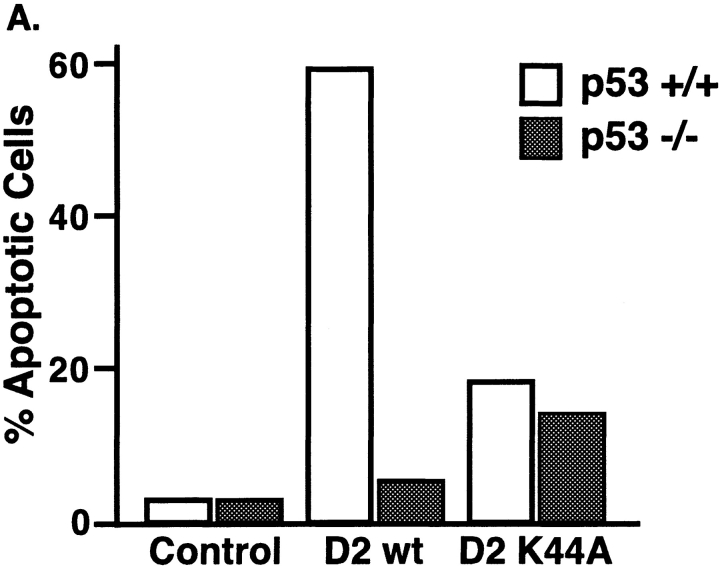
Figure 7.
Dynamin-2–induced apoptosis is p53 dependent. (A) p53+/+ and p53−/− mouse embryo fibroblasts were infected with the indicated adenoviruses and incubated for 24 h before staining with the fluorescent DNA dye YO-PRO-1 (1 μM). Quantification of the number of early apoptotic cells (i.e., YO-PRO-1 positive), expressed as the percentage of viable cells (i.e., PI negative) is shown at 24 h postinfection. 100 cells were counted for each condition. (B) p53ts cells were grown at 37 or 32°C for 24 h and then infected with the indicated adenoviruses and incubated for a further 24 h at the same temperature. MTT conversion assays were used to measure cell proliferation and viability. The results are expressed as percent of uninfected control ± SD (n = 3).
In a different approach, we confirmed that p53 function as a transcription regulator is required for dynamin-2–induced apoptosis. Akata cells, a p53-deficient Burkitt Lymphoma cell line (Farrell et al. 1991; Takada 1984), were coinfected with Ad-tTA and Ad-Dyn2wt. Like the p53−/− cells, Akata cells showed no discernible cytotoxicity, even at 48 h (not shown). To test for a functional requirement for p53, we obtained Akata cells that had been stably transformed with a temperature-sensitive mutant of p53 (V143A, designated p53ts; Chen et al. 1998). This mutation is located within the core domain of p53 and interferes with its DNA binding ability and activity as a sequence specific transcription activator (Zhang et al. 1994; Chen et al. 1998). These cells were infected with Ad-tTA alone or together with Ad-Dyn2wt and maintained at either 37°C, the nonpermissive temperature, or 32°C, the permissive temperature for p53 function. When compared with uninfected and Ad-tTA–infected control cells, p53ts cells grown at the nonpermissive temperature expressing inactive p53 (white bars) were completely resistant to the cytotoxic effects of dyn2 expression (Fig. 7 B). In contrast, cells grown at the permissive temperature expressing active p53 (shaded bars) were significantly more sensitive to overexpression of dyn2wt leading to reduced cell proliferation as quantitated by MTT conversion assays. Dyn2 expression in all Akata cell lines was confirmed by Western blot analysis and immunofluorescence (data not shown).
Together with the finding of dyn2wt-induced p53 nuclear translocation and our inability to detect cell cycle arrest, these results suggest that dynamin-2 is part of a signaling pathway that can activate the transcription factor p53 and potentially trigger apoptosis.
Discussion
Dynamin plays an essential role in receptor-mediated endocytosis via clathrin-coated pits. Here we present new evidence that the ubiquitously expressed isoform, dynamin-2, functions as a signal transducing GTPase with the potential to induce a p53-dependent apoptotic phenotype. Only small increases (≤5-fold) in intracellular dyn2wt expression are required to trigger apoptosis. Dynamin-triggered apoptosis is not due to alterations in endocytic membrane trafficking. Even at high levels of overexpression, dyn2wt, which induces apoptosis, has no effect on endocytosis (Altschuler et al. 1998; Kasai et al. 1999) and neither dyn1K44A nor dyn2K44A, which inhibit endocytosis, induces apoptosis. The finding that dyn2K44A, which is defective in GTP binding, does not trigger apoptosis argues that induction of apoptosis reflects an increase in the levels of dyn2·GTP rather than of the protein per se. These data are consistent with dynamin's function as a regulatory GTPase.
In our experimental system, we have increased the cellular levels of dyn2·GTP through overexpression of the wild-type molecule. As for other regulatory GTPases, we imagine that cellular levels of dyn2·GTP are physiologically controlled by upstream events that signal either increased GTP binding or decreased rates of intrinsic GTP hydrolysis. Interestingly, dynamin encodes its own intramolecular GAP, which is activated by self-assembly to stimulate GTP hydrolysis (Sever et al. 1999). Our finding that dyn2ΔPRD is significantly more potent in activating caspase-3 may be explained in the context of this intramolecular regulation of dynamin GTPase activity. Dynamin's PRD is required for membrane targeting (Shpetner et al. 1996) and is a positive regulator of dynamin self-assembly and assembly-stimulated GTPase activity (Warnock et al. 1997; Warnock et al. 1996). Thus, it is possible that dyn2ΔPRD will exhibit reduced assembly-stimulated GAP activity in vivo and, therefore, exist in its GTP-bound state for prolonged times relative to dyn2wt. Alternatively, or in addition, SH3-domain containing partners of dynamin-2, which are known to stimulate dynamin's GTPase activity in vitro (Gout et al. 1993; Herskovits et al. 1993), might act to regulate the intracellular levels of dyn2·GTP such that in the absence of these interactions dyn2ΔPRD would be more potent.
The observed apoptotic phenotype is highly specific for wild-type dynamin-2; the neuronal isoform dynamin-1 does not induce apoptosis. While dynamin-2 has a higher propensity for self-assembly and a higher intrinsic rate of GTP hydrolysis than dynamin-1 (Warnock et al. 1997), these are unlikely to account for the specificity we observe. First, these enzymological differences are only ∼3-fold, while dynamin-2 is >50-fold more potent at inducing apoptosis than dynamin-1. Second, the enzymological differences between dynamin-1 and dynamin-2 are eliminated by removal of the PRD (Warnock et al. 1997), yet dyn2ΔPRD is more potent than dyn2wt at activating caspase-3.
We have established that a modest (≤5-fold) increase in endogenous dynamin-2 levels can trigger a signaling pathway leading to p53 activation and p53-dependent apoptosis. However, we do not know whether this signaling pathway is activated by endogenous levels of dynamin-2. For example, it remains possible that the apoptotic phenotype is an indirect effect of the overexpression of dyn2wt disturbing a tightly regulated balance of interacting molecules or pathways. The transcription factor p53 is known to respond to oncogenic and genotoxic stress induced by a variety of factors. Thus, while our data demonstrate that dynamin-2 functions as a signaling GTPase capable of affecting transcriptional regulation, the apoptotic response observed under our experimental conditions may not be the physiologically relevant signaling pathway.
Dynamin has been shown to interact with many signaling molecules, including grb2 (Gout et al. 1993), c-src (Ahn et al. 1999), βγ-subunits (Lin and Gilman 1996), and ERK kinase (Earnest et al. 1996). Although the functional significance of these interactions has not been explored, it is possible that some or all of these partners might be upstream or downstream in a dynamin-2 signaling pathway. In this regard, recent evidence has suggested that dominant-negative dynamin mutants inhibit MAP kinase signaling independent of their effects on endocytosis (Kranenburg et al. 1999; Whistler and von Zastrow 1999). Moreover, overexpression of dynamin-2 wild-type potentiates MAP kinase activation in response to receptor stimulation (Kranenburg et al. 1999). More work needs to be done to identify the physiological signals upstream of dynamin-2 and the direct downstream effectors that make up this dynamin-2–specific signaling pathway(s).
Dynamin-2 activity as a signaling GTPase can occur independently of any measurable effects on endocytosis. However, it seems likely under physiological conditions that these two functions are linked. Recent findings have suggested that dynamin functions in endocytosis as a regulatory GTPase controlling downstream effectors that mediate vesicle formation (Sever et al. 1999). In its capacity as a regulatory GTPase governing membrane dynamics at the cell surface, dynamin may respond to physiological changes at the plasma membrane to modulate the rate of endocytosis or to trigger signaling cascades that affect transcriptional regulation. Interestingly, other components of the endocytic machinery, Eps15 (Salcini et al. 1997) and epsin (Hyman et al. 2000) have recently been linked to transcriptional regulation, thus strengthening the idea that pathways are in place to alter cell physiology in response to events at the plasma membrane.
Given the importance of the mitochondria in apoptosis, it is noteworthy that the only other dynamin family members in C. elegans and Drosophila are located on the mitochondrial membrane where they regulate membrane dynamics (Smirnova et al. 1998; van der Bliek 1999). These proteins are functionally and structurally homologous to mgm1p and dnm1p in S. cerevisiae (Otsuga et al. 1998; Shepard and Yaffe 1999) and share greatest homology with dynamin in the GTPase and GED domains (van der Bliek 1999). While it is difficult to link these functionally diverse dynamin family members to vesicular trafficking, each of them is associated with membrane dynamics at critical cellular organelles: the plasma membrane and the mitochondria. Perhaps these dynamin family members share common functions as monitors of membrane integrity and homeostasis, coupled to their role as regulators of membrane dynamics at their respective locations.
Mammalian dynamin-2, but not dynamin-1, triggers apoptosis, raising the evolutionary question: did dynamin-2 gain its apoptosis-signaling capability or did dynamin-1 lose it? As we have shown for mammalian dynamin-2, high concentrations of wild-type C. elegans and Drosophila dynamin DNA were also found to be toxic in their respective transgenic animals (van der Bliek, A.M., personal communication). Dynamin is expressed at 50–100-fold higher levels in neurons than in other tissues in C. elegans (Clark et al. 1997), Drosophila (Chen et al. 1992), and in mammals (Terlecky, L.J., and S.L. Schmid, unpublished results). Thus, it is reasonable to assume that mammalian dynamin-1 might have lost its ability to induce apoptosis in order to achieve these high levels of expression and instead gained activity as a highly efficient and more integral component of the tightly regulated endocytic machinery responsible for synaptic vesicle recycling. Given that dynamin's PRD, which is most divergent between isoforms, is not required for induction of apoptosis, the structural features that functionally distinguish dynamin-1 from dynamin-2 remain to be identified.
Supplemental Material
Acknowledgments
We wish to thank Dale Warnock and Shana Barbas for initiating our studies of the dynamin-2 isoform, Joe Trotter for his help with the FACS analysis, Cecilia Subauste for help with the caspase assay, Geoffrey Wahl for providing the p53+/+ and p53−/− mouse fibroblasts, Weiping Chen for providing the p53-deficient and p53ts AKATA cells, and Yoram Altschuler for the Ad-tTA virus. We also acknowledge Weiping Chen and members of the Schmid lab for helpful discussion.
This work was supported by National Institutes of Health grants GM42455 and CA58689 to S.L. Schmid and GM19689 to K.N. Fish. This is TSRI manuscript number 12909-CB.
Footnotes
The online version of this article contains supplemental material.
Abbreviations used in this paper: Ad, adenovirus; dyn, dynamin; moi, multiplicity of infection; pfu, plaque forming unit; PRD, proline/arginine-rich domain; tTA, tetracycline-responsive transcription activator.
References
- Ahn S., Maudsley S., Luttrell L.M., Lefkowitz R.J., Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 1999;274:1185–1188. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- Altschuler Y., Barbas S.M., Terlecky L.J., Tang K., Hardy S., Mostov K.E., Schmid S.L. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C., Davis J.B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Blagosklonny M.V. Loss of function and p53 protein stabilization. Oncogene. 1997;15:1889–1893. doi: 10.1038/sj.onc.1201374. [DOI] [PubMed] [Google Scholar]
- Budihardjo I., Holt O., Lutter M., Luo X., Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Burns T.F., El-Deiry W.S. The p53 pathway and apoptosis. J. Cell Physiol. 1999;181:231–239. doi: 10.1002/(SICI)1097-4652(199911)181:2<231::AID-JCP5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chen M.S., Burgess C.C., Vallee R.B., Wadsworth S.C. Developmental stage- and tissue-specific expression of shibire, a Drosophila gene involved in endocytosis. J. Cell Sci. 1992;103:619–628. doi: 10.1242/jcs.103.3.619. [DOI] [PubMed] [Google Scholar]
- Chen W., Huang S., Cooper N.R. Levels of p53 in Epstein-Barr virus-infected cells determine cell fateapoptosis, cell cycle arrest at the G1/S boundary without apoptosis, cell cycle arrest at the G2/M boundary without apoptosis, or unrestricted proliferation. Virology. 1998;251:217–226. doi: 10.1006/viro.1998.9431. [DOI] [PubMed] [Google Scholar]
- Choisy-Rossi C., Reisdorf P., Yonish-Rouach E. Mechanisms of p53-induced apoptosisin search of genes which are regulated during p53-mediated cell death. Toxicol. Lett. 1998;102–103:491–496. doi: 10.1016/s0378-4274(98)00238-0. [DOI] [PubMed] [Google Scholar]
- Clark S.G., Shurland D.L., Meyerowitz E.M., Bargmann C.I., van der Bliek A.M. A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans . Proc. Natl. Acad. Sci. USA. 1997;94:10438–10443. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H., Baba T., Warnock D.E., Schmid S.L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H., Freundlieb S., Gossen M., Bujard H., Schmid S.L. Tightly regulated and inducible expression of a dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 1995;257:209–221. doi: 10.1016/s0076-6879(95)57026-8. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Juan G., Li X., Gorczyca W., Murakami T., Traganos F. Cytometry in cell necrobiologyanalysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- Ding H.F., Fisher D.E. Mechanisms of p53-mediated apoptosis. Crit. Rev. Oncog. 1998;9:83–98. doi: 10.1615/critrevoncog.v9.i1.60. [DOI] [PubMed] [Google Scholar]
- Earnest S., Khokhlatchev A., Albanesi J.P., Barylko B. Phosphorylation of dynamin by ERK2 inhibits the dynamin-microtubule interaction. FEBS Lett. 1996;396:62–66. doi: 10.1016/0014-5793(96)01074-5. [DOI] [PubMed] [Google Scholar]
- Evan G., Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- Farrell P.J., Allan G.J., Shanahan F., Vousden K.H., Crook T. p53 is frequently mutated in Burkitt's lymphoma cell lines. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:2879–2887. doi: 10.1002/j.1460-2075.1991.tb07837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish K.N., Depto A.S., Moses A.V., Britt W., Nelson J.A. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J. Virol. 1995;69:3737–3743. doi: 10.1128/jvi.69.6.3737-3743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout I., Dhand R., Hiles I.D., Fry M.J., Panayotou G., Das P., Truong O., Totty N.F., Hsuan J., Booker G.W. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75:25–36. [PubMed] [Google Scholar]
- Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Hardy S., Kitamura M., Harris-Stansil T., Dai Y., Phipps M.L. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits J.S., Burgess C.C., Obar R.A., Vallee R.B. Effects of mutant rat dynamin on endocytosis. J. Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw J.E., Schmid S.L. Dynamin self assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- Hyman J., Chen H., Di Fiore P.P., De Camilli P., Brunger A.T. Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH2-terminal homology (ENTH) domain, structurally similar to armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn(2)+ finger protein (PLZF) J. Cell Biol. 2000;149:537–546. doi: 10.1083/jcb.149.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C.E., Schrier R., Ghazal P., Wiley C., Nelson J.A. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 1991;65:6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idziorek T., Estaquier J., De Bels F., Ameisen J.C. YO-PRO-1 permits cytofluorimetric analysis of programmed cell death (apoptosis) without interfering with cell viability. J. Immunol. Methods. 1995;185:249–258. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- Jones S.N., Sands A.T., Hancock A.R., Vogel H., Donehower L.A., Linke S.P., Wahl G.M., Bradley A. The tumorigenic potential and cell growth characteristics of p53-deficient cells are equivalent in the presence or absence of Mdm2. Proc. Natl. Acad. Sci. USA. 1996;93:14106–14111. doi: 10.1073/pnas.93.24.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K., Shin H.W., Shinotsuka C., Murakami K., Nakayama K. Dynamin II is involved in endocytosis but not in the formation of transport vesicles from the trans-Golgi network. J. Biochem. 1999;125:780–789. doi: 10.1093/oxfordjournals.jbchem.a022349. [DOI] [PubMed] [Google Scholar]
- Kranenburg O., Verlaan I., Moolenaar W.H. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J. Biol. Chem. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- Lane D.P. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lane D.P., Hall P.A. MDM2-arbiter of p53's destruction. Trends Biochem. Sci. 1997;22:372–374. doi: 10.1016/s0968-0004(97)01119-5. [DOI] [PubMed] [Google Scholar]
- Levine A.J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lin H.C., Gilman A.G. Regulation of dynamin I GTPase activity by G protein betagamma subunits and phosphatidylinositol 4,5 bisphosphate. J. Biol. Chem. 1996;271:27979–27982. doi: 10.1074/jbc.271.45.27979. [DOI] [PubMed] [Google Scholar]
- McNiven M.A. Dynamina molecular motor with pinchase action. Cell. 1998;94:151–154. doi: 10.1016/s0092-8674(00)81414-2. [DOI] [PubMed] [Google Scholar]
- McNiven M.A., Cao H., Pitts K.R., Yoon Y. The dynamin family of mechanoenzymespinching in new places. Trends Biochem. Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- McPake C., Shetty S., Kitchingman G., Harris L. Wild-type p53 induction mediated by replication-deficient adenoviral vectors. Cancer Res. 1999;59:4247–4251. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survivalapplication to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Otsuga D., Keegan B.R., Brisch E., Thatcher J.W., Hermann G.J., Bleazard W., Shaw J.M. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J. Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot M., Zhang Y.Z., Kramer J.A., Wells K.S., Jones L.J., Hanzel D.K., Lugade A.G., Singer V.L., Haugland R.P. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J. Histochem. Cytochem. 1996;44:1363–1372. doi: 10.1177/44.12.8985128. [DOI] [PubMed] [Google Scholar]
- Prives C. Signaling to p53breaking the MDM2-p53 circuit. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- Salcini A.E., Confalonieri S., Doria M., Santolini E., Tassi E., Minenkova O., Cesareni G., Pelicci P.G., Di Fiore P.P. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S.L., McNiven M.A., De Camilli P. Dynamin and its partnersa progress report. Curr. Opin. Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- Sever S., Muhlberg A.B., Schmid S.L. Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature. 1999;398:481–486. doi: 10.1038/19024. [DOI] [PubMed] [Google Scholar]
- Sever S., Damke H., Schmid S.L. Garrotes, springs, ratchets and whipsputting dynamin models to the test. Traffic. 2000;1:385–392. doi: 10.1034/j.1600-0854.2000.010503.x. [DOI] [PubMed] [Google Scholar]
- Shepard K.A., Yaffe M.P. The yeast dynamin-like protein, Mgm1p, functions on the mitochondrial outer membrane to mediate mitochondrial inheritance. J. Cell Biol. 1999;144:711–720. doi: 10.1083/jcb.144.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpetner H.S., Herskovits J.S., Vallee R.B. A binding site for SH3 domain targets dynamin to coated pits. J. Biol. Chem. 1996;271:13–16. doi: 10.1074/jbc.271.1.13. [DOI] [PubMed] [Google Scholar]
- Simpson F., Hussain N.K., Qualmann B., Kelly R.B., Kay B.K., McPherson P.S., Schmid S.L. SH3-domain-containing proteins function at the distinct steps in clathrin-coated vesicle formation. Nature Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- Smirnova E., Shurland D.L., Ryazantsev S.N., van der Bliek A.M. A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E., Shurland D.L., Newman-Smith E.D., Pishvaee B., van der Bliek A.M. A model for dynamin self-assembly based on binding between three different protein domains. J. Biol. Chem. 1999;274:14942–14947. doi: 10.1074/jbc.274.21.14942. [DOI] [PubMed] [Google Scholar]
- Stowell M.H.B., Marks B., Wigge P., McMahon H.T. Nucleotide-dependent conformational changes in dynaminevidence for a mechanochemical molecular spring. Nature Cell Biol. 1999;1:27–32. doi: 10.1038/8997. [DOI] [PubMed] [Google Scholar]
- Streblow D.N., Soderberg-Naucler C., Vieira J., Smith P., Wakabayashi E., Ruchti F., Mattison K., Altschuler Y., Nelson J.A. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99:511–520. doi: 10.1016/s0092-8674(00)81539-1. [DOI] [PubMed] [Google Scholar]
- Takada K. Cross-linking of cell surface immunoglobulins induces Epstein-Barr viurs in Burkitt lymphoma cell lines. Int. J. Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A., Lazebnik Y. Caspasesenemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Urrutia R., Henley J.R., Cook T., McNiven M.A. The dynaminsRedundant or distinct functions for an expanding family of related GTPases? Proc. Natl. Acad. Sci. USA. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A.M. Functional diversity in the dynamin family. Trends Cell Biol. 1999;9:96–102. doi: 10.1016/s0962-8924(98)01490-1. [DOI] [PubMed] [Google Scholar]
- van der Bliek A.M., Redelmeier T.E., Damke H., Tisdale E.J., Meyerowitz E.M., Schmid S.L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D.E., Schmid S.L. Dynamin GTPase, a force generating molecular switch. Bioessays. 1996;18:885–893. doi: 10.1002/bies.950181107. [DOI] [PubMed] [Google Scholar]
- Warnock D.E., Terlecky L.J., Schmid S.L. Dynamin GTPase is stimulated by crosslinking through the C terminal proline rich domain. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:1322–1328. doi: 10.1002/j.1460-2075.1995.tb07118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D.E., Hinshaw J.E., Schmid S.L. Dynamin self assembly stimulates its GTPase activity. J. Biol. Chem. 1996;271:22310–22314. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- Warnock D.E., Baba T., Schmid S.L. Ubiquitously expressed dynamin-II has a higher intrinsic GTPase activity and a greater propensity for self-assembly than neuronal dynamin-I. Mol. Biol. Cell. 1997;8:2553–2562. doi: 10.1091/mbc.8.12.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler J.L., von Zastrow M. Dissociation of functional roles of dynamin in receptor-mediated endocytosis and mitogenic signal transduction. J. Biol. Chem. 1999;274:24575–24578. doi: 10.1074/jbc.274.35.24575. [DOI] [PubMed] [Google Scholar]
- Zhang W., Guo X.Y., Hu G.Y., Liu W.B., Shay J.W., Deisseroth A.B. A temperature-sensitive mutant of human p53. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:2535–2544. doi: 10.1002/j.1460-2075.1994.tb06543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.