Abstract
In higher eukaryotes, phospholipid and cholesterol synthesis occurs mainly in the endoplasmic reticulum, whereas sphingomyelin and higher glycosphingolipids are synthesized in the Golgi apparatus. Lipids like cholesterol and sphingomyelin are gradually enriched along the secretory pathway, with their highest concentration at the plasma membrane. How a cell succeeds in maintaining organelle-specific lipid compositions, despite a steady flow of incoming and outgoing transport carriers along the secretory pathway, is not yet clear. Transport and sorting along the secretory pathway of both proteins and most lipids are thought to be mediated by vesicular transport, with coat protein I (COPI) vesicles operating in the early secretory pathway. Although the protein constituents of these transport intermediates are characterized in great detail, much less is known about their lipid content. Using nano-electrospray ionization tandem mass spectrometry for quantitative lipid analysis of COPI-coated vesicles and their parental Golgi membranes, we find only low amounts of sphingomyelin and cholesterol in COPI-coated vesicles compared with their donor Golgi membranes, providing evidence for a significant segregation from COPI vesicles of these lipids. In addition, our data indicate a sorting of individual sphingomyelin molecular species. The possible molecular mechanisms underlying this segregation, as well as implications on COPI function, are discussed.
Keywords: COPI-coated vesicles, sphingomyelin, cholesterol, lipid sorting, nano-electrospray ionization tandem mass spectrometry
Introduction
The various compartments of eukaryotic cells possess unique lipid compositions. The molecular mechanisms that maintain these steady-state distributions are largely unknown (van Meer 1989; Pelham 1996). Most membrane lipids are synthesized in the ER and the Golgi apparatus (Bishop and Bell 1988; van Meer 1989), and are then transported to their final destinations (van Meer 1993). A lipid gradient exists for most lipids along the organelles of the secretory pathway (van Meer 1998). Lipids such as sphingomyelin (SM) and cholesterol are found only in low amounts in the ER, and are enriched at the plasma membrane, whereas the Golgi complex represents an intermediate between ER and plasma membrane. An SM and cholesterol gradient seems to exist also within the Golgi complex, with slightly higher concentrations of these lipids towards the trans side (Orci et al. 1981; Pagano et al. 1989; Coxey et al. 1993; Cluett and Machamer 1996; Cluett et al. 1997).
It is generally assumed that net transport of lipids occurs mainly via vesicular transport (Pagano 1990; Trotter and Voelker 1994; van Helvoort and van Meer 1995; Rothman and Wieland 1996). According to this model, both cell surface proteins and lipids would use the same kind of intracellular carriers. However, in the case of some lipids, like cholesterol, a nonvesicular pathway seems to play a major role in transport (for a review see Liscum and Munn 1999). Two types of vesicular carriers have been implicated in protein transport in the early secretory pathway: coat protein (COP)I-coated vesicles, which are thought to function in bidirectional transport within the Golgi complex (Orci et al. 1986, Orci et al. 1989, Orci et al. 1997; Ostermann et al. 1993; Nickel et al. 1998; Warren and Malhotra 1998; Volchuk et al. 2000) and retrograde transport from the Golgi apparatus to the ER (Cosson and Letourneur 1994, Cosson and Letourneur 1997; Letourneur et al. 1994; Lowe and Kreis 1998; Nickel and Wieland 1998; Pelham 1998), and COPII-coated vesicles, which mediate export of newly synthesized proteins from the ER (Barlowe et al. 1994; Kuge et al. 1994; Aridor et al. 1995; Kuehn and Schekman 1997; Bannykh et al. 1998; Barlowe 1998). SM is synthesized in the Golgi apparatus (Futerman et al. 1990; Jeckel et al. 1990; Allan and Obradors 1999), and thus COPI-coated vesicles are potentially involved in anterograde transport of this membrane lipid towards the TGN. Similarly, the ER is the export site of newly synthesized cholesterol (Reinhart et al. 1987; Krisans 1992), implying that both COPII- and COPI-coated vesicles may be involved in its cell surface delivery. Various kinds of transport intermediates have been implicated in TGN to cell surface transport (Keller and Simons 1997), some of which may also be involved in the delivery of SM and cholesterol from the TGN to the cell surface. Interestingly, a difference in interleaflet clear space between COPI vesicles and their donor membranes has been reported that might be caused by a change in lipid composition during budding (Orci et al. 1996), for example, by a reduced amount of SM and cholesterol (van Meer 1998) in COPI vesicles.
We have analyzed the lipid composition of COPI-coated vesicles with respect to phosphatidylcholine (PC), as it is the bulk lipid of eukaryotic membranes, along with SM and cholesterol, which are enriched at the cell surface under steady-state conditions (van Meer 1998). As reported previously, nano-electrospray ionization tandem mass spectrometry (nano-ESI-MS/MS) provides a powerful tool to quantitatively assess the lipid composition of biological membranes in a highly sensitive manner (Brügger et al. 1997; Sandhoff et al. 1999). This method has allowed us to study even the minute amounts of highly purified COPI-coated vesicles generated from isolated Golgi membranes in vitro and to quantitatively compare their lipid content with their parental membranes. We have exploited class-specific fragmentation to selectively detect and quantify distinct classes of lipids by precursor ion scanning (PREC).
Here we show that COPI-coated vesicles contain significantly less SM and cholesterol compared with Golgi donor membranes, providing evidence for a segregation from COPI vesicles of these lipids. The purity of the subcellular fractions was analyzed by both biochemical and morphological means to assess the significance of our quantitative analysis. Segregation from COPI-coated vesicles of both SM and cholesterol has implications for the function of these transport carriers, the mode of net transport of these cell surface lipids, and the functional organization of the Golgi apparatus.
Materials and Methods
Materials
1,2-O-dilauroyl-sn-glycero-3-phosphocholine, 1,2-O-dimyristoyl-sn-glycero-3-phosphocholine, 1,2-O-diarachidoyl-sn-glycero-3-phosphocholine, and 1,2-O-dibehenoyl-sn-glycero-3-phosphocholine were purchased from Avanti Polar Lipids, Inc. N-oleoyl-SM, N-sphingosyl-phosphorylcholine, myristic acid, and pentacosanoic acid were obtained from Sigma-Aldrich. All solvents were HPLC grade and purchased from J.T. Baker. GTPγS and GTP were obtained from Roche Molecular Biochemicals. 3,4-[13C2]cholesterol was purchased from Cambridge Isotope Laboratories, Inc. Sulfur trioxide pyridine complex and 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline were obtained from Fluka. Glass capillaries for nano-ESI-MS/MS were purchased from Teer Coatings, Ltd. Silica gel 60 (40–63 μm) and RP-18 silica gel (40–63 μm) were obtained from Merck. α-MEM, penicillin, streptomycin, l-glutamine, trypsin-EDTA, and PBS for cell culture were purchased from Biochrom. FCS was obtained from PAA Laboratories GmbH. Mouse mAb against β-COP (M3A5) was purchased from Sigma-Aldrich. A rabbit antibody to p23 (no. 1327) was used as described (Sohn et al. 1996).
Cell Culture
CHO cells were grown in α-MEM containing 10% (vol/vol) heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine at 37°C and 5% CO2.
Subcellular Fractionation
CHO Golgi membrane fractions were prepared by a modification of the method of Balch et al. 1984. All procedures were carried out at 4°C. In a standard preparation, 8 liters of CHO cells (density of 5 × 105 cells/ml) were harvested by centrifugation (10 min at 500 g av), washed twice with PBS (10 min at 500 g av), twice with homogenization buffer (250 mM sucrose in 10 mM Tris-HCl, pH 7.4, 4°C) (10 min at 1,500 g av), and finally resuspended in four volumes of homogenization buffer. Cells were homogenized by passing 15 times through a ball-bearing homogenizer. The homogenate was brought to a sucrose concentration of 37% (wt/wt) by addition of 62% (wt/wt) sucrose in 10 mM Tris-HCl, pH 7.4. EDTA was added to a final concentration of 1 mM. 12 ml of this solution was placed in one SW 28 tube and overlaid with 15 ml 35% (wt/wt) sucrose in 10 mM Tris-HCl, pH 7.4, and 9 ml 29% (wt/wt) sucrose in 10 mM Tris-HCl, pH 7.4. Gradients were centrifuged for 2.5 h at 25,000 rpm. Typically, ∼2 ml of a Golgi-enriched membrane fraction was recovered at the 35%–29% sucrose interphase. For further purification, membranes of five gradients were collected, diluted 1:3 with 10 mM Tris-HCl, pH 7.4, and pelleted onto a 50% (wt/wt) sucrose cushion in an SW 28 tube (60 min at 25,000 rpm). Membranes were adjusted to 45% (wt/wt) sucrose in 10 mM Tris-HCl, pH 7.4 (in 1.5 ml), and overlaid with a step gradient of 1.5 ml of 40% (wt/wt), 35% (wt/wt), 30% (wt/wt), 25% (wt/wt), 20% (wt/wt), and 15% (wt/wt) sucrose in 10 mM Tris-HCl, pH 7.4. After centrifugation of the gradient (18 h at 36,000 rpm, SW 41 rotor), 30 fractions were collected from bottom to top and analyzed for marker enzyme activities. The fraction with the highest activity of Golgi marker enzyme was frozen in liquid nitrogen and stored at −80°C.
Rat liver Golgi membranes were prepared as described by Warren and colleagues (Slusarewicz et al. 1994) and further purified on a continuous sucrose gradient, as described above for CHO Golgi membranes.
Marker Enzyme Analysis
Enzymatic assays for alkaline phosphodiesterase (plasma membrane), NADH cytochrome c reductase (ER), and β-N-acetylglucosaminidase (lysosomes and late endosomes) were carried out as described by Warnock et al. 1993. SM synthase (Golgi apparatus) was assayed according to Jeckel et al. 1992. Early endosome enrichment in the homogenate and the Golgi-enriched fraction was determined by Western blot analysis, which quantified the amount of syntaxin 13 (rat liver) or transferrin receptor (CHO). Quantitative evaluation was performed using NIH Image software.
Bovine Brain Cytosol
Bovine brain cytosol was prepared by a modification of the method described by Malhotra et al. 1989. All procedures were carried out at 4°C. In brief, meninges, blood vessels, and white matter were removed from three fresh bovine brains. 120 ml breaking buffer (25 mM Tris-HCl, pH 7.4 at 4°C, 250 mM sucrose, 500 mM KCl, 2 mM EGTA, 1 mM DTT, 1 mM PMSF, 0.5 mM 1,10-phenanthroline, 2 μM pepstatin A, 2 μg/ml aprotinin, and 0.5 μg/ml leupeptin) was added to 100 g of brain. Brains were homogenized in a Waring blender (two times for 30 s at low speed, then 30 s at high speed). After centrifugation of the homogenate (60 min at 12,000 rpm in a GS-3 rotor), the supernatant was centrifuged in TFT 55.38 tubes for 90 min at 44,000 rpm. The supernatant was concentrated by a factor of about eight in a Militan ultra concentration unit (Millipore), dialyzed two times against 30 liters of dialysis buffer (25 mM Tris-HCl, pH 7.4, 50 mM KCl, 1 mM DTT), and centrifuged again for 90 min at 44,000 rpm in TFT 55.38 tubes. The supernatant was frozen in liquid nitrogen and stored at −80°C.
Purification of COPI-coated Vesicles
COPI-coated vesicles were prepared from CHO Golgi membranes or rat liver Golgi membranes, as described by Serafini and Rothman 1992, with a final concentration of 63 μg/ml Golgi membranes and 2.4 mg/ml (CHO COPI vesicles) or 4.8 mg/ml (rat liver COPI vesicles) bovine brain cytosol. The final continuous sucrose gradient was fractionated from the bottom into 18 fractions of 250 μl each. A 25-μl aliquot of each fraction was precipitated with three volumes of chloroform/methanol (1:2, vol/vol), and pellets were resuspended in SDS-PAGE sample buffer, and subjected to 13% SDS-PAGE. Proteins were transferred to a PVDF membrane and probed with antibodies against p23 and β-COP. The peak vesicle fractions (typically, fractions 6–8) were pooled, frozen in liquid nitrogen, and stored at −80°C.
For comparison of COPI vesicles generated either in the presence of GTP or GTPγS, COPI vesicle generations were performed, as described, except that the vesicles were directly released from pelleted Golgi membranes and loaded on top of a continuous sucrose gradient. Assay volumes were reduced to 15 ml. A final concentration of either 1 mM GTP or 25 μM GTPγS was used.
EM
Samples were fixed in 2.5% glutaraldehyde and the membranes were pelleted for 60 min at 45,000 rpm and 4°C in a TLA 45 rotor. The pellets were processed for conventional EM, as described previously (Lannert et al. 1998). COPI-coated vesicle fractions were fixed in the presence of 75 μg BSA in PBS.
Protein Determination
The concentration of protein was determined with a BCA protein assay kit (Pierce Chemical Co.), using BSA as the standard protein.
SM Standard Synthesis
Synthesis of N-myristoyl-SM and N-pentacosanoyl-SM was performed under argon. Glassware was flame-dried before use. N-sphingosyl-phosphorylcholine (21.5 μmol) was dissolved in a solution of either myristic acid or pentacosanoic acid (9.56 mM) in 4.5 ml ethanol. After addition of 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline (EEDQ) (50 μmoles), the reaction mixture was stirred for 7 h at 50°C. EEDQ (50 μmol) was added a second time and the solution was stirred for an additional 12 h at room temperature. The reaction mixture was concentrated under reduced pressure and the crude product was subjected to flash chromatography (silica gel, 11.5 × 2-cm column; the eluent was composed of toluene/diethylether/methanol/water, 3:3:3.5:0.7, vol/vol/vol/vol). Product-containing fractions were pooled as monitored by TLC (silica gel; the running solvent was composed of toluene/diethylether/methanol/water, 3:3:4:1, vol/vol/vol/vol), concentrated under reduced pressure, redissolved in 1.7 ml chloroform/methanol (1:2, vol/vol), and subjected to a second flash chromatography (RP-18 silica gel, 10 × 1-cm column). The column was preequilibrated with methanol/water (1:1, vol/vol). After loading of the product, the column was washed six times with 5 ml water, then six times with 5 ml methanol/water (1:1, vol/vol). Elution was performed stepwise with 5 ml methanol, 15 ml chloroform/methanol (1:1, vol/vol), 15 ml chloroform/methanol (2:1, vol/vol), and 10 ml chloroform. Product-containing fractions (monitored by TLC) were dried under a gentle stream of nitrogen, dissolved in 100 ml chloroform/methanol (1:2, vol/vol), and stored under argon at −20°C. Phosphate was determined as described (Rouser et al. 1970). Products were verified by mass spectrometric analysis. Typically, a yield of 50–60% was achieved.
Lipid Analysis
Lipids were extracted from membrane fractions with chloroform/methanol/HCl (50:100:1.5, vol/vol/vol), as described by Bligh and Dyer 1959. Using a gentle stream of nitrogen, the organic phase was evaporated and the dried lipids were redissolved in chloroform/methanol (1:2, vol/vol). Phosphate determination was performed as described (Rouser et al. 1970), except that the assay volume was reduced by a factor of four. Glassware was used throughout the procedures.
MS was done on a QII triple quadrupole instrument (Micromass) equipped with a nano-ESI source. Nitrogen was used as a drying gas. The source temperature was set to 30°C. A capillary voltage of ± 600–800 V was applied, depending on the ion mode. Argon was used as collision gas at a nominal pressure of 3 × 10−3 mbar. Resolution of Q1 and Q3 was set to achieve isotope resolution. A collision energy of 30 eV was used for PC and SM detection in positive PREC mode, selecting a fragment ion of mass/charge (m/z) 184 (phosphocholine ion). Detection of cholesterol as cholesterol sulfate was done in negative PREC mode, selecting for a fragment ion of m/z 97 (sulfate ion) at a collision energy of 62 eV (Sandhoff et al. 1999). The mass range m/z scanned was 600–1,000 for PC and SM detection and 450–480 for cholesterol sulfate detection. For each quantitative measurement, 100 (PC and SM) or 50 (cholesterol) consecutive scans of 4-s duration were averaged.
PC and SM quantification was done as described (Brügger et al. 1997). 1,2-O-dilauroyl-sn-glycero-3-phosphocholine, 1,2-O-dimyristoyl-sn-glycero-3-phosphocholine, 1,2-O-diarachidoyl-sn-glycero-3-phosphocholine, and 1,2-O-dibehenoyl-sn-glycero-3-phosphocholine, as well as N-myristoyl-SM, N-oleoyl-SM, and N-pentacosanoyl-SM were used as standards. In brief, PC and SM standards dissolved in chloroform/methanol (1:2, vol/vol) were added to the extraction solvent before lipid extraction of membrane fractions. Dried lipids were redissolved in chloroform/methanol (1:2, vol/vol). Ammonium acetate (100-mM stock solution in methanol) was added to a final concentration of 5 mM to acidify the solution. Before mass spectrometric analysis, samples were spun at 15,000 g av for 5 min at 4°C in a microfuge (Eppendorf). Nano flow borosilicate glass tips of type D (Teer Coatings) were used. Instrument parameters were set as described above. For quantitative analysis, mass correction and isotope correction for [M+1], [M+2], and [M+3] isotopes were performed. Quantification of cholesterol was performed as described previously (Sandhoff et al. 1999).
Results
Characterization of Subcellular Fractions
CHO Golgi membrane preparations were performed as described by Balch et al. 1984, and Golgi membranes were further purified by flotation in a continuous sucrose gradient. Relative enrichment of Golgi membranes and the degree of cross-contamination with other subcellular organelles were determined by measuring the distribution of marker enzymes for Golgi (SM synthase), ER (NADH-cytochrome c reductase), plasma membrane (alkaline phosphodiesterase), lysosomes/late endosomes (β-N-acetylglucosaminidase), and early endosomes (syntaxin 13 for rat liver cells or transferrin receptor for CHO cells). Enrichment of marker proteins is expressed as the ratio of specific enzyme activity (units/mg protein) in the Golgi-enriched fractions and in the homogenate. Typically, the resulting Golgi fraction was 52-fold enriched in the Golgi marker, 3-fold enriched in the plasma membrane marker, 4-fold enriched in the lysosomes/late endosomes marker, 7-fold enriched in early endosomes/plasma membrane, and 0.9-fold enriched in the ER marker (Table ). Isolation of rat liver Golgi membranes was performed as described previously (Slusarewicz et al. 1994), and the Golgi-enriched fraction was then further purified by applying a continuous sucrose flotation gradient. As shown in Table , fractions taken for lipid analysis were characterized by a 97-fold enrichment of a Golgi marker, a 5-fold enrichment of a plasma membrane marker, a 3-fold enrichment of a lysosomes/late endosomes marker, an 11-fold enrichment of an early endosome marker, and a 1.2-fold enrichment of an ER marker.
Table 1.
Characterization of Donor Golgi Membranes by Marker Enzyme Analysis
| Organelle | CHO Golgienrichment(fold/homogenate) | Rat liver Golgienrichment(fold/homogenate) |
|---|---|---|
| Golgi | 52.2 | 96.9 |
| Plasma membrane | 3.2 | 4.7 |
| Lysosome/late endosome | 3.9 | 2.9 |
| Early endosomes | 7.2 | 10.5 |
| ER membrane | 0.9 | 1.2 |
CHO and rat liver Golgi membranes were isolated as described in Materials and Methods. For marker enzyme analyses, specific activities (expressed as enzyme activity per milligram of protein) of crude homogenate fractions (containing membrane proteins as well as cytosolic proteins) and Golgi-enriched membrane fractions were compared. The enrichment of marker enzymes is presented as the ratio of specific enzyme activities determined in the Golgi fractions and specific activities measured in the homogenate fractions.
In addition to marker protein analysis, both CHO Golgi- (Fig. 1 A) and rat liver Golgi-enriched membrane fractions (Fig. 1 B) that were used as donor membranes for vesicle generation were analyzed by EM. This morphological analysis revealed that these fractions are highly enriched in cisternal membranes.
Figure 1.
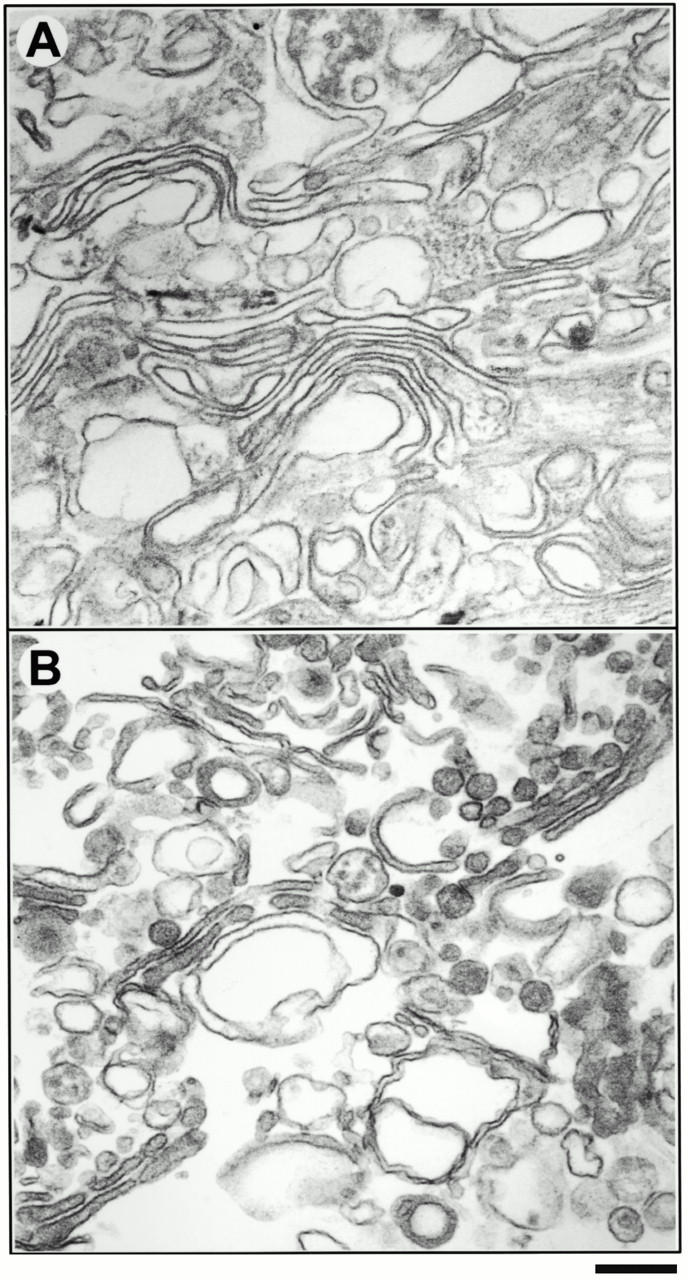
Morphology of CHO and rat liver Golgi membranes by EM. CHO and rat liver Golgi-enriched membranes were isolated and processed for EM, as described in the Materials and Methods. (A) Conventional Epon sections of a CHO Golgi fraction and (B) a rat liver Golgi fraction are shown. Bars: (A) 200 nm, (B) 210 nm.
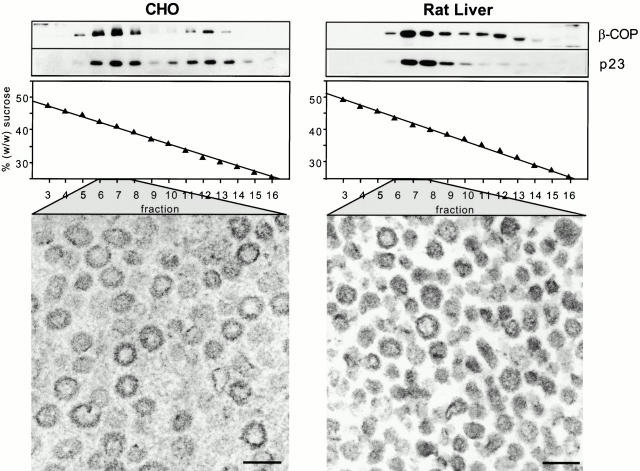
COPI vesicles were generated in vitro as described (Serafini and Rothman 1992). Vesicle preparations were analyzed for the presence of two abundant COPI vesicle proteins: β-COP, a subunit of the membrane-associated coat protein complex coatomer (Duden et al. 1991; Serafini et al. 1991), and p23, an integral membrane protein enriched in COPI vesicles (Sohn et al. 1996). COPI vesicles typically band at a buoyant density of ∼1.18 g/cm3 (41%, wt/wt, sucrose) (Serafini et al. 1991), corresponding to fraction 7 of the sucrose gradient that represents the final purification step. As shown in Fig. 2 (top left, CHO vesicle gradients; top right, rat liver vesicle gradients), both proteins comigrate in this part of the gradient in a single peak. EM of COPI vesicle preparations (fractions 6–8 of the continuous sucrose gradient) derived from both CHO (bottom left) and rat liver Golgi membranes (bottom right) revealed a high degree of homogeneity.
Figure 2.
Characterization of in vitro–generated COPI-coated vesicles. Transport vesicles were generated from either CHO (left) or rat liver (right) Golgi membranes in the presence of bovine brain cytosol, ATP-regenerating system, and GTPγS. COPI vesicles were purified on a continuous sucrose gradient. The gradients were fractionated from the bottom into 18 fractions. Aliquots of fractions 3–16 of the isopycnic gradients were chloroform/methanol-precipitated, and analyzed by running on a 13% SDS-PAGE and Western blotting, using antibodies against β-COP and p23 (top). COPI vesicles are recovered in fractions 6–8, corresponding to an average sucrose concentration of 41% (wt/wt). Below are electron micrographs of COPI-coated vesicles present in fractions 6–8. Bars, 90 nm.
Lipid Analysis of Subcellular Fractions by Nano-ESI-MS/MS
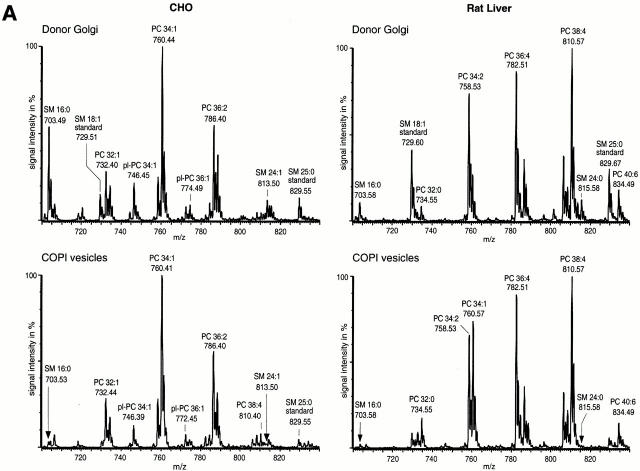
We have shown that nano-ESI-MS/MS is a powerful tool for lipid analysis of biological membranes at the low picomole level (Brügger et al. 1997; Sandhoff et al. 1999). For quantitative analyses, lipid extractions were performed in the presence of internal lipid standards. Lipid species were chosen as standards that are not present in endogenous membrane extracts, as described in the Materials and Methods. Quantification of PC and SM was performed in the PREC mode. PREC allows specific detection of only those molecular ions in which collision-induced fragmentation results in a fragment ion of a selected m/z ratio. Fragmentation of PC and SM leads to a positively charged cholinephosphate fragment ion (m/z 184). Therefore, specific detection of PC and SM was performed in the positive ion mode by scanning for precursors of m/z 184 (PREC 184). Fig. 3 A shows representative spectra of PREC 184 scans for CHO Golgi membranes (top left), CHO COPI vesicles (bottom left), rat liver Golgi membranes (top right), and rat liver COPI vesicles (bottom right). The highest peak in the mass range displayed was set to 100%. Since both PC and SM have only one positive charge, the given m/z ratios represent their molecular masses. Each group (a group is defined by an identical number of C atoms in both fatty acid chains, with varying amounts of double bonds) of PC or SM molecular species is clearly separated from the next group carrying an additional (CH2)2 unit. Signals between the main PC groups originate from plasmalogen PCs. Since no appropriate standard lipids for plasmalogen PCs are available at this time, plasmalogens were not included in this quantification. When both CHO- and rat liver–derived COPI vesicles were compared with their parental membranes, a significant decrease of SM species in relation to PC species was observed. To determine whether this change is due to an increase of PC and/or a decrease of SM, lipid quantitation was performed.
Figure 3.
(A) Mass spectrometric analysis of donor Golgi membranes and Golgi-derived COPI-coated vesicles. Golgi membranes were isolated from either CHO cells or rat liver and incubated with bovine brain cytosol, ATP-regenerating system, and GTPγS. Vesicles were purified on an isopycnic sucrose gradient. As described in Materials and Methods, lipids from donor Golgi membranes and COPI vesicles were extracted in the presence of internal PC and SM standards. PC and SM quantification was performed in PREC 184 mode. The top panels show PREC 184 spectra of CHO (left) and rat liver (right) donor Golgi membranes. The bottom panels show the corresponding PREC 184 spectra of COPI vesicles generated from CHO (left) and rat liver (right) Golgi membranes. Each spectrum shown was averaged from 100 separate scans, each of 4-s duration. The molecular masses in daltons, the corresponding numbers of total carbon (C) atoms in both fatty acids, and the numbers of double bonds (Σ C atoms:Σ of double bonds) are given for the major peaks. pl, plasmalogen. (B) Quantification of PC, SM, and cholesterol in donor Golgi and COPI vesicle fractions of CHO or rat liver membranes. Lipid quantification was performed as described in Materials and Methods. The results of quantification of PC (black bars), SM (white bars), and cholesterol (gray bars) in CHO (top) and in rat liver (bottom) membranes are shown. The amounts of PC, SM, and cholesterol in donor Golgi membranes are set to 100%. Results are shown as mean ± SD. Reference to absolute amounts of these lipids present in COPI vesicles and their parental membrane fractions is given in Table .
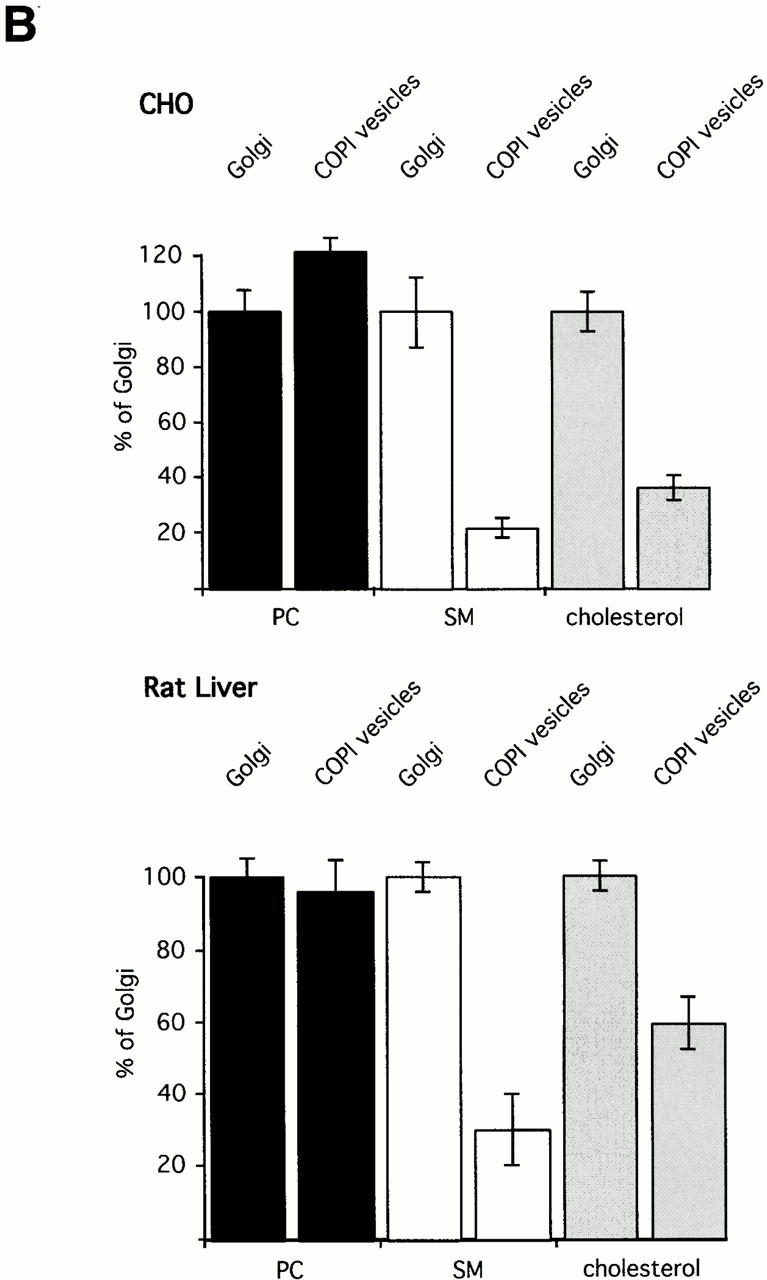
SM and Cholesterol Are Segregated from COPI-coated Vesicles
To allow a direct quantitative comparison of lipids in isolated COPI vesicles and their Golgi donor membranes, the absolute amounts of SM, PC, and cholesterol in each fraction need to be determined. To this end, total lipid phosphate was determined, as well as PC, SM, and cholesterol. Calculation of the protein to phospholipid ratio of Golgi membranes and of COPI vesicles revealed an increase of the protein to phospholipid ratio in COPI vesicles by a factor of about four compared with Golgi donor membranes. This increase reflects the abundance of cytosolic coat proteins on the surface of COPI vesicles, as well as the small inner diameter of the vesicles, and explains the higher buoyant density of COPI vesicles compared with their parental membranes.
As shown in Table , quantitative analysis of CHO Golgi membranes revealed ∼37 mol% PC and ∼14 mol% SM of total phospholipid, and a molar ratio of cholesterol to phospholipid of ∼0.72. In rat liver Golgi membranes, PC constituted ∼57 mol% and SM ∼5 mol% of total phospholipid. A molar ratio of cholesterol to phospholipid of ∼0.15 was determined. While the lipid composition of rat liver Golgi membranes is well reported in the literature, no corresponding data exist for CHO Golgi membranes. The lipid compositions for rat liver Golgi fractions were found to be 55 mol% PC, 8 mol% SM, and a cholesterol to phospholipid ratio of 0.2 (van Meer 1998), in good agreement with the above data obtained by nano-ESI-MS/MS analysis.
Table 2.
Quantitative Lipid Analysis of Donor Golgi
| Membrane | PC | SM | Cholesterol |
|---|---|---|---|
| mol% of phospholipid | molChol/molPi | ||
| CHO Golgi | 37.1 ± 3.0 (n = 6) | 14.3 ±1.8 (n = 6) | 0.720 ± 0.058 (n = 2) |
| CHO COPI vesicles | 45.0 ± 2.0 (n = 6) | 3.0 ± 0.5 (n = 6) | 0.264 ± 0.036 (n = 2) |
| Rat liver Golgi | 57.2 ± 3.0 (n = 2) | 5.0 ± 0.2 (n = 2) | 0.153 ± 0.020 (n = 2) |
| Rat liver COPI vesicles | 55.0 ± 5.0 (n = 3) | 1.5 ± 0.5 (n = 3) | 0.096 ± 0.012 (n = 2) |
Golgi membranes and COPI vesicles were isolated as described in Materials and Methods. Phosphate determination was performed on membrane fractions. Quantification of lipids was performed in the presence of internal lipid standards by nano-ESI-MS/MS, as described in Materials and Methods. SM and PC values are given as the mol% of total phospholipid; cholesterol values are expressed as the ratio of moles of cholesterol to moles of total phospholipid (Pi). Data are expressed as mean ± SD; n is the number of experiments.
The quantification of PC, SM, and cholesterol in CHO Golgi-derived COPI vesicles revealed a contribution of ∼45 mol% PC and ∼3 mol% SM to total phospholipid (Table ). The cholesterol to phospholipid ratio was found to be ∼0.26. Analysis of COPI vesicles derived from rat liver Golgi donor membranes revealed a contribution of ∼55 mol% PC and ∼1.5 mol% SM to total phospholipid, and a cholesterol to phospholipid ratio of ∼0.09. The relative changes between donor Golgi membranes and COPI vesicles in the contribution of PC, SM, and cholesterol to total phospholipid are given in Fig. 3 B. For better comparison, the value of each lipid in donor Golgi membranes was set to 100%. Absolute values are given in Table . In CHO COPI vesicles, a slight increase was determined in PC by a factor of 1.2 compared with CHO Golgi membranes, whereas the amount of PC in rat liver Golgi membranes and rat liver COPI vesicles was not significantly changed. In contrast, for both CHO and rat liver COPI vesicles, a decrease in the amount of SM was observed. SM is segregated from the vesicles by a factor of 4.8 (CHO) and by a factor of 3.3 (rat liver). A similar observation was made for cholesterol. The amounts of cholesterol in Golgi-derived COPI-coated vesicles are 2.7 times lower than in CHO Golgi membranes. Similarly, the amounts of cholesterol in rat liver COPI vesicles are 1.7 times lower than in rat liver Golgi membranes. Thus, about four cholesterol molecules are segregated together with each SM molecule from CHO COPI vesicles, and two cholesterol molecules are segregated with each SM molecule from rat liver COPI vesicles.
GTP Hydrolysis Does Not Artificially Cause Lipid Segregation during COPI Budding
Previously, it was shown that cargo uptake into COPI vesicles requires ADP ribosylation factor (ARF)-mediated hydrolysis of GTP (Nickel et al. 1998; Malsam et al. 1999; Pepperkok et al. 2000). To address the question of whether lipid sorting is also affected by GTP hydrolysis, we compared the lipid patterns of COPI vesicles generated in the presence of either GTP or GTPγS. As the amounts of vesicles obtained in the presence of GTP are extremely low, a phosphate determination of GTP vesicles is virtually not feasible. Therefore, we used the PC to SM ratio in donor Golgi and vesicle fractions as a measure for lipid sorting. Preparations of COPI vesicles in the presence of either GTPγS or GTP were performed as described in Materials and Methods. Lipid extraction of vesicle preparations was performed in the presence of internal standards for SM and PC. Both lipids were detected via PREC 184 scanning. The resulting spectra are shown in Fig. 4. Compared with parental Golgi membranes, we determined a PC to SM ratio that was 2.13 ± 0.02–fold higher in GTPγS-derived vesicles and 2.12 ± 0.01–fold higher in GTP-derived vesicles. Compared with vesicle preparations performed according to the standard protocol (Serafini and Rothman 1992), the apparent segregation factors are smaller due to a lower purity of the vesicle preparation.
Figure 4.
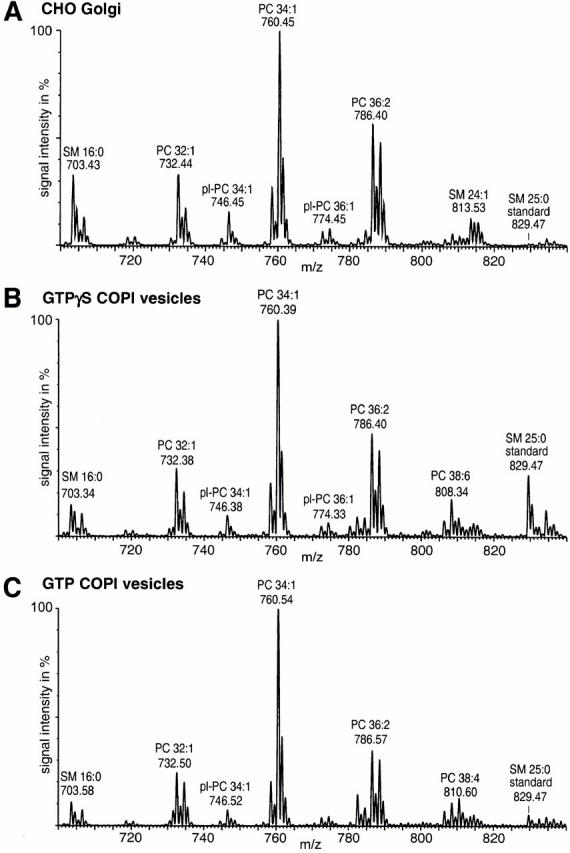
Generation of COPI vesicles from CHO Golgi membranes in the presence of either GTPγS or GTP. Preparations of COPI vesicles were performed as described in the Materials and Methods. Lipid quantification of the various membrane fractions was done after addition of internal PC and SM standards before extraction. Quantification was performed as described. Illustrated are PREC 184 spectra of (A) CHO Golgi donor membranes, (B) COPI vesicles from GTPγS incubations, and (C) COPI vesicles from GTP incubations. pl, plasmalogen.
In conclusion, lipid segregation is not restricted to GTPγS-COPI vesicles and, therefore, appears to be physiologically relevant.
Relative Distribution of PC and SM Species in Golgi Donor Membranes and COPI Vesicles
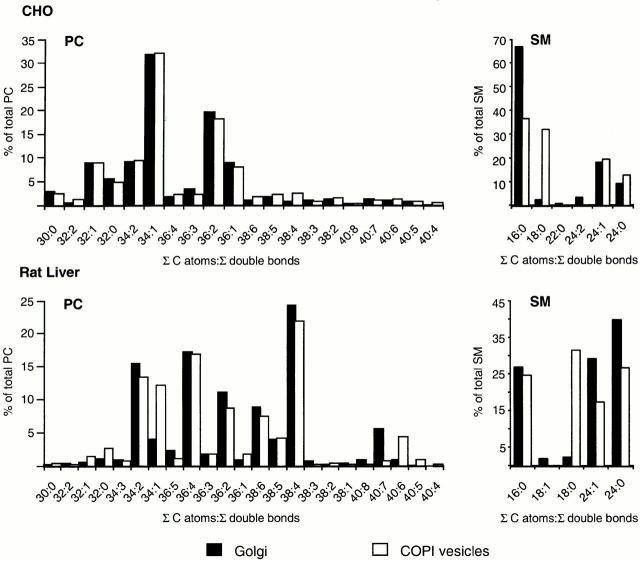
Besides changes in the absolute amounts of PC and SM in Golgi membranes and COPI-coated vesicles, respectively, we were interested in changes in the relative distribution of the various PC and SM species. As illustrated in Fig. 5, in CHO Golgi membranes and in CHO COPI vesicles, the overall pattern of PC molecular species is very similar. In rat liver Golgi membranes and rat liver COPI vesicles, a significant change in the amount of PC 34:1 was observed. This lipid was increased from 3.9% in the Golgi membrane to 12.2% in COPI vesicles. Regarding the relative amounts of the various SM species in donor membranes and vesicles, drastic changes were observed in both CHO and rat liver COPI vesicles. SM 18:0 was enriched by a factor of 12 in CHO COPI vesicles, and by a factor of 13 in rat liver COPI vesicles. Whereas this increase of SM 18:0 was accompanied by a decrease in SM species with shorter fatty acid chains (i.e., SM 16:0) in CHO COPI vesicles, SM species with longer fatty acid chains (i.e., SM 24:0 and SM 24:1) were found to be reduced in rat liver COPI vesicles.
Figure 5.
Comparison of fatty acid composition of SM and PC species in Golgi membranes and Golgi-derived COPI-coated vesicles. Lipid quantification was performed as described in Materials and Methods. The results obtained from the CHO system are depicted at the top; those of the rat liver system are shown at the bottom. The relative amounts of PC (left) and SM (right) species derived from Golgi donor membranes (black bars) were compared with those of COPI-coated vesicles (white bars). Data are expressed as percentage of each lipid molecular species (identical total number of C atoms in both fatty acids as well as identical number of total double bonds, indicated as Σ C atoms:Σ double bonds) of PC or SM to total amount of PC or SM, respectively.
Discussion
This study represents the first qualitative and quantitative lipid analysis of mammalian Golgi-derived COPI-coated transport vesicles. Using nano-ESI-MS/MS, we demonstrate that only low levels of the plasma membrane-enriched lipids SM and cholesterol are present in this type of transport intermediate. Moreover, in comparison to the parental Golgi membranes, we provide evidence that these lipids are segregated during budding, resulting in a significant change of the PC to SM and the PC to cholesterol ratios, respectively.
Determination of the Extent of Lipid Segregation
While COPI-coated vesicles can be purified to near homogeneity, the corresponding Golgi donor membranes contain contaminations of other organelle membranes such as ER, plasma membranes, and endosomes/lysosomes. Therefore, differences in the apparent lipid compositions of Golgi preparations and COPI vesicle preparations could be due, in part, to the greater purity of the latter. To assess the significance of the observed lipid segregation, the purity of Golgi donor membranes was characterized at both the biochemical and the ultrastructural level. In addition, Golgi-enriched fractions were obtained from two different sources, which are characterized by different degrees of individual organelle contaminations. This also allowed us to judge on the generality of the phenomenon described. Based on data providing the relative contributions of individual organelles to total cellular membranes (Griffiths et al. 1989; McDowall et al. 1989; Alberts et al. 1994) and on the factors of enrichment of the various contaminating membranes in CHO and rat liver Golgi preparations, we determined the absolute contamination of the Golgi fraction with other organelles (Table ). Whereas plasma membranes and endosomal/lysosomal membranes are the main contaminations in our CHO Golgi preparations (representing a total of 9.7%), rat liver Golgi membranes are mainly contaminated by ER membranes (8.1%). As demonstrated by the following calculation for CHO membranes, contaminating membranes with a lower content of SM (ER) and those with a higher content of SM (plasma membrane and endosomes/lysosomes) roughly compensate for each other. To assess the influence on the measured value for SM in donor fractions of contaminating membranes, we used the data given in Table , in conjunction with the SM contents reported for plasma membranes and endosomes/lysosomes (14–18 mol%) (Cezanne et al. 1992; Warnock et al. 1993), as well as ER membranes (10 mol%) (Blough et al. 1977; Brotherus and Renkonen 1977; Urade et al. 1988). The calculations were made based on an SM content of 18 mol% for plasma membranes, the highest value reported. The actual SM content of CHO Golgi membranes present in our preparations would then be 14.1 mol%, a rather minor difference compared with the overall SM content (14.3 mol%, see Table ) measured in this membrane preparation. (Since no data were available for the SM content of CHO-derived ER membranes, data obtained from lipid analysis of Chinese hamster V79-UF and BHK cells were averaged. It is of note that this high SM value chosen might be an overestimation. With respect to the calculations, a lower SM value would have increased the determined SM value of the Golgi donor fraction. In general, SM and cholesterol seem to be present in cell culture cells in higher amounts (relative to total lipid) than in tissue-derived membranes.)
Table 3.
Relative Contribution of Various Organelles to Total Membrane Surface Area of Donor Golgi Fractions
| Organelle | CHO Golgi fraction | Rat liver Golgi fraction |
|---|---|---|
| Golgi | 85.8 | 89.9 |
| ER | 4.5 | 8.1 |
| Plasma membrane | 5.9 | 1.2 |
| Lysosomes/endosomes | 3.8 | 0.8 |
Relative contributions of Golgi, ER, plasma membrane, and lysosomes/endosomes to total membrane surface area of purified Golgi fractions were calculated by combining data presenting the percentage of each organelle to the total membrane surface area (as reported in the literature) with the enrichment factors of marker enzymes determined for these organelles.
Another possibility explaining the observed lipid segregation is that COPI budding might be restricted to vesicular tubular clusters and the early Golgi apparatus. This was suggested based on a predominant cis-Golgi steady-state localization of coatomer (Oprins et al. 1993). Vesicular tubular clusters and cis-Golgi membranes are characterized by an SM and cholesterol content lower than the late Golgi apparatus. However, this does not necessarily reflect a higher rate of COPI vesicle formation, since coatomer might play an additional structural role at the intermediate compartment (IC)/cis-Golgi interface (Elazar et al. 1994). Moreover, a quantitative immunoelectron microscopy study directly analyzing the occurrence of buds and vesicles in close proximity to the various Golgi domains revealed that COPI vesicles appear to form from all individual cisternae of a stack to a similar extent (Orci et al. 1997). Nevertheless, the determined values for SM and cholesterol in COPI vesicles are even lower than those reported for the IC/cis-Golgi network (CGN). Based on lipid analysis of viral envelope membranes, Cluett and Machamer 1996 determined in HeLa cells an SM content of 8% (of total phospholipid) in the CGN and of 11% in the TGN, thus a 1.4-fold enrichment towards the TGN. For BHK-21 cells, an SM content of ∼5–10 mol% in IC/CGN membranes, of ∼18.7% in the TGN, and of ∼12.8% in the total Golgi membrane preparation were determined (Cluett et al. 1997). Thus, the amount of SM, even in IC/CGN membranes (on average 7.6%, see above), is higher than that determined for COPI vesicles (3% in CHO and 1.5% in rat liver COPI vesicles).
GTP Hydrolysis Does Not Affect Lipid Segregation
As demonstrated previously, ARF-mediated GTP hydrolysis is a prerequisite for uptake of cargo into COPI vesicles (Nickel et al. 1998; Malsam et al. 1999; Pepperkok et al. 2000). Therefore, we compared the PC to SM ratio (a measure for lipid sorting) of COPI vesicles, generated in the presence of either GTP or GTPγS, with that of CHO Golgi membranes. In both cases, a very similar value was obtained, indicative of a physiological relevance of the observed lipid segregation.
Segregation of Molecular Species
The profile of the individual molecular species of PC revealed no significant differences when CHO Golgi donor membranes and CHO COPI vesicles were compared. In both membrane fractions, PC 34:1 (mainly 16:0/18:1) was the major PC species, accounting for 32% of the total PC. In rat liver Golgi membranes, PC 34:1 (mainly 16:0/18:1) contributed only 3.9% of the total PC, but was enriched by a factor of three in COPI-coated vesicles (12.2% of the total PC). This increase was not accompanied by the decrease of any particular PC species. A change of the overall ratio of saturated to unsaturated PC species was not observed. Since saturated PC species are reported to be enriched in the plasma membrane (as opposed to the ER) (Keenan and Morre 1970), COPI-coated vesicle–mediated lipid sorting does not appear to contribute to this steady-state distribution. Most likely, remodeling of lipid species accounts for the higher degree of lipid saturation at the plasma membrane (MacDonald and Sprecher 1991; Schmid et al. 1991; Schneiter et al. 1999). The relative distribution of the various SM species in donor membranes and COPI vesicles was found to be significantly changed in both the CHO and the rat liver system. N-stearoyl-SM was enriched in COPI vesicles by a factor of 12 (CHO) and 13 (rat liver). In CHO Golgi-derived COPI-coated vesicles, this increase was accompanied by a decrease of SM species with shorter fatty acyl chains (e.g., SM 16:0), whereas SM species with longer fatty acyl chains (e.g., SM 24:0) were reduced in rat liver COPI vesicles. Further work is needed to elucidate the function of lipid species sorting during COPI-coated vesicle biogenesis.
Possible Mechanisms of Lipid Segregation
How are SM and cholesterol segregated from COPI-coated vesicles? Two possibilities for the observed lipid segregation can be envisaged: (a) a dynamic segregation of lipids concomitant with COPI-coated vesicle formation that is based on the molecular mechanism of vesicle budding, or (b) segregation before the budding process, resulting in the formation of COPI-coated vesicles from membrane subdomains poor in SM and cholesterol. A dynamic segregation during the budding process might be caused by preferential interactions of certain membrane phospholipids with parts of the protein machinery that mediates COPI vesicle budding. Both coat proteins (i.e., ARF and/or coatomer), COPI coat receptors (Stamnes et al. 1995; Sohn et al. 1996; Bremser et al. 1999), and other factors might potentially be involved in these kinds of interactions. Alternatively, SM and cholesterol may be mainly organized in SM- and cholesterol-enriched microdomains (Simons and Ikonen 1997; Brown and London 1998) representing Golgi substructures that do not function as donor sites for COPI vesicle biogenesis. There is evidence that such a microdomain exists in the early Golgi (Gkantiragas, I., B. Brügger, E. Stüven, K. Löhr, F. Lottspeich, F.T. Wieland, and J.B. Helms, manuscript submitted for publication).
Transport of SM and Cholesterol
SM and cholesterol are known to form concentration gradients along the organelles of the secretory pathway, with the highest enrichment in the plasma membrane. Since ER membranes are poor in SM and cholesterol, their segregation from COPI vesicles might be interpreted in a way that favors an exclusive role of these vesicles in retrograde transport. While some data support this view (Bannykh and Balch 1997; Mironov et al. 1997; Gaynor et al. 1998), there is strong evidence for a direct role of COPI in anterograde transport (Orci et al. 1986, Orci et al. 1989, Orci et al. 1997; Ostermann et al. 1993; Nickel et al. 1998; Volchuk et al. 2000; for a review see Warren and Malhotra 1998). In addition, the small amounts of SM and cholesterol remaining in COPI vesicles may well be sufficient for an anterograde net transport of these lipids, depending on the rate of transport and turnover at the plasma membrane. Alternatively, these lipids might be transported by other carriers towards the plasma membrane. Indeed, a direct transport route between ER and plasma membrane, bypassing the Golgi, was suggested for cholesterol (Kaplan and Simoni 1985; Urbani and Simoni 1990; Liscum and Dahl 1992; Smart et al. 1996; Uittenbogaard et al. 1998; Heino et al. 2000; for reviews see Anderson 1998; Liscum and Munn 1999).
In conclusion, application of nano-ESI-MS/MS has, for the first time, allowed us to quantitate the lipid content of highly purified COPI vesicles. This analysis provides evidence for a lipid segregation in Golgi membranes, as indicated by a significant reduction of SM and cholesterol content in COPI-coated vesicles compared with their parental membranes.These data are consistent with a reduced interleaflet clear space in the membrane of COPI-coated vesicles compared with Golgi membranes (Orci et al. 1996). Elucidation of the underlying mechanism as well as the determination of the lipid compositions of other vesicular carriers will be major challenges for future studies and will result in a deeper understanding of lipid sorting.
Acknowledgments
We thank Heidi McBride (EMBL, Heidelberg) for providing α-syntaxin 13 antibody. We also thank the members of the Wieland lab for helpful discussions throughout the work, Gabriele Weiss for excellent technical assistance, and Thomas Weber (Mount Sinai School of Medicine, New York, NY), James A. McNew and Christine A. Hughes (Memorial Sloan-Kettering Cancer Center, New York, NY) for critical comments on the manuscript.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 352) and the Human Frontier Science Program Organization (HFSPO).
Footnotes
J. Malsam's present address is Department of Cell Biology, Yale University School of Medicine, 333 Cedar St., New Haven, CT 06520-8002.
Abbreviations used in this paper: ARF, ADP ribosylation factor; CGN, cis-Golgi network; COP, coat protein; IC, intermediate compartment; m/z, mass/charge; nano-ESI-MS/MS, nano-electrospray ionization tandem mass spectrometry; PC, phosphatidylcholine; PREC, precursor ion scanning; SM, sphingomyelin.
References
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Robert, and J.D. Watson. 1994. Intracellular compartments and protein sorting. In Molecular Biology of the Cell. Garland Publishing, Inc., New York. 551–598.
- Allan D., Obradors M.J. Enzyme distributions in subcellular fractions of BHK cells infected with Semliki forest virusevidence for a major fraction of sphingomyelin synthase in the trans-Golgi network. Biochim. Biophys. Acta. 1999;1450:277–287. doi: 10.1016/s0167-4889(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Anderson R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Aridor M., Bannykh S.I., Rowe T., Balch W.E. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J. Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W.E., Dunphy W.G., Braell W.A., Rothman J.E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bannykh S.I., Balch W.E. Membrane dynamics at the endoplasmic reticulum–Golgi interface. J. Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh S.I., Nishimura N., Balch W.E. Getting into the Golgi. Trends Cell Biol. 1998;8:21–25. doi: 10.1016/s0962-8924(97)01184-7. [DOI] [PubMed] [Google Scholar]
- Barlowe C. COPII and selective export from the endoplasmic reticulum. Biochim. Biophys. Acta. 1998;1404:67–76. doi: 10.1016/s0167-4889(98)00047-0. [DOI] [PubMed] [Google Scholar]
- Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M.F., Ravazzola M., Amherdt M., Schekman R. COPIIa membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bishop W.R., Bell R.M. Assembly of phospholipids into cellular membranesbiosynthesis, transmembrane movement and intracellular translocation. Annu. Rev. Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blough H.A., Tiffany J.M., Aaslestad H.G. Lipids of rabies virus and BHK-21 cell membranes. J. Virol. 1977;21:950–955. doi: 10.1128/jvi.21.3.950-955.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremser M., Nickel W., Schweikert M., Ravazzola M., Amherdt M., Hughes C.A., Sollner T.H., Rothman J.E., Wieland F.T. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Brotherus J., Renkonen O. Phospholipids of subcellular organelles isolated from cultured BHK cells. Biochim. Biophys. Acta. 1977;486:243–253. doi: 10.1016/0005-2760(77)90020-0. [DOI] [PubMed] [Google Scholar]
- Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Brügger B., Erben G., Sandhoff R., Wieland F.T., Lehmann W.D. Quantitative analysis of biological membrane lipids at the low picomole level by nano electrospray ionization tandem mass spectrometry. Proc. Natl. Acad. Sci. USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cezanne L., Navarro L., Tocanne J.F. Isolation of the plasma membrane and organelles from Chinese hamster ovary cells. Biochim. Biophys. Acta. 1992;1112:205–214. doi: 10.1016/0005-2736(92)90393-z. [DOI] [PubMed] [Google Scholar]
- Cluett E.B., Machamer C.E. The envelope of vaccinia virus reveals an unusual phospholipid in Golgi complex membranes. J. Cell Sci. 1996;109:2121–2131. doi: 10.1242/jcs.109.8.2121. [DOI] [PubMed] [Google Scholar]
- Cluett E.B., Kuismanen E., Machamer C.E. Heterogeneous distribution of the unusual phospholipid semilysobisphosphatidic acid through the Golgi complex. Mol. Biol. Cell. 1997;8:2233–2240. doi: 10.1091/mbc.8.11.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P., Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Cosson P., Letourneur F. Coatomer (COPI)-coated vesiclesrole in intracellular transport and protein sorting. Curr. Opin. Cell Biol. 1997;9:484–487. doi: 10.1016/s0955-0674(97)80023-3. [DOI] [PubMed] [Google Scholar]
- Coxey R.A., Pentchev P.G., Campbell G., Blanchette-Mackie E.J. Differential accumulation of cholesterol in Golgi compartments of normal and Niemann-Pick type C fibroblasts incubated with LDLa cytochemical freeze-fracture study. J. Lipid Res. 1993;34:1165–1176. [PubMed] [Google Scholar]
- Duden R., Griffiths G., Frank R., Argos P., Kreis T.E. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Elazar Z., Orci L., Ostermann J., Amherdt M., Tanigawa G., Rothman J.E. ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J. Cell Biol. 1994;124:415–424. doi: 10.1083/jcb.124.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A.H., Stieger B., Hubbard A.L., Pagano R.E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J. Biol. Chem. 1990;265:8650–8657. [PubMed] [Google Scholar]
- Gaynor E.C., Graham T.R., Emr S.D. COPI in ER/Golgi and intra-Golgi transportdo yeast COPI mutants point the way? Biochim. Biophys. Acta. 1998;1404:33–51. doi: 10.1016/s0167-4889(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Back R., Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J. Cell Biol. 1989;109:2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino S., Lusa S., Somerharju P., Ehnholm C., Olkkonen V.M., Ikonen E. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl. Acad. Sci. USA. 2000;97:8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Birk R., Schmidt R.R., Wieland F. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett. 1990;261:155–157. doi: 10.1016/0014-5793(90)80659-7. [DOI] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Burger K.N., van Meer G., Wieland F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J. Cell Biol. 1992;117:259–267. doi: 10.1083/jcb.117.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M.R., Simoni R.D. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J. Cell Biol. 1985;101:446–453. doi: 10.1083/jcb.101.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T.W., Morre D.J. Phospholipid class and fatty acid composition of Golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry. 1970;9:19–25. doi: 10.1021/bi00803a003. [DOI] [PubMed] [Google Scholar]
- Keller P., Simons K. Post-Golgi biosynthetic trafficking. J. Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Krisans S.K. The role of peroxisomes in cholesterol metabolism. Am. J. Respir. Cell Mol. Biol. 1992;7:358–364. doi: 10.1165/ajrcmb/7.4.358. [DOI] [PubMed] [Google Scholar]
- Kuehn M.J., Schekman R. COPII and secretory cargo capture into transport vesicles. Curr. Opin. Cell Biol. 1997;9:477–483. doi: 10.1016/s0955-0674(97)80022-1. [DOI] [PubMed] [Google Scholar]
- Kuge O., Dascher C., Orci L., Rowe T., Amherdt M., Plutner H., Ravazzola M., Tanigawa G., Rothman J.E., Balch W.E. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J. Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannert H., Gorgas K., Meissner I., Wieland F.T., Jeckel D. Functional organization of the Golgi apparatus in glycosphingolipid biosynthesis. Lactosylceramide and subsequent glycosphingolipids are formed in the lumen of the late Golgi. J. Biol. Chem. 1998;273:2939–2946. doi: 10.1074/jbc.273.5.2939. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E.C., Hennecke S., Demolliere C., Duden R., Emr S.D., Riezman H., Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Liscum L., Dahl N.K. Intracellular cholesterol transport. J. Lipid Res. 1992;33:1239–1254. [PubMed] [Google Scholar]
- Liscum L., Munn N.J. Intracellular cholesterol transport. Biochim. Biophys. Acta. 1999;1438:19–37. doi: 10.1016/s1388-1981(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Lowe M., Kreis T.E. Regulation of membrane traffic in animal cells by COPI. Biochim. Biophys. Acta. 1998;1404:53–66. doi: 10.1016/s0167-4889(98)00046-9. [DOI] [PubMed] [Google Scholar]
- MacDonald J.I., Sprecher H. Phospholipid fatty acid remodeling in mammalian cells. Biochim. Biophys. Acta. 1991;1084:105–121. doi: 10.1016/0005-2760(91)90209-z. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Serafini T., Orci L., Shepherd J.C., Rothman J.E. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Malsam J., Gommel D., Wieland F.T., Nickel W. A role for ADP ribosylation factor in the control of cargo uptake during COPI-coated vesicle biogenesis. FEBS Lett. 1999;462:267–272. doi: 10.1016/s0014-5793(99)01543-4. [DOI] [PubMed] [Google Scholar]
- McDowall A., Gruenberg J., Romisch K., Griffiths G. The structure of organelles of the endocytic pathway in hydrated cryosections of cultured cells. Eur. J. Cell Biol. 1989;49:281–294. [PubMed] [Google Scholar]
- Mironov A.A., Weidman P., Luini A. Variations on the intracellular transport themematuring cisternae and trafficking tubules. J. Cell Biol. 1997;138:481–484. doi: 10.1083/jcb.138.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W., Wieland F.T. Biosynthetic protein transport through the early secretory pathway. Histochem. Cell Biol. 1998;109:477–486. doi: 10.1007/s004180050249. [DOI] [PubMed] [Google Scholar]
- Nickel W., Malsam J., Gorgas K., Ravazzola M., Jenne N., Helms J.B., Wieland F.T. Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPγS in vitro. J. Cell Sci. 1998;111:3081–3090. doi: 10.1242/jcs.111.20.3081. [DOI] [PubMed] [Google Scholar]
- Oprins A., Duden R., Kreis T.E., Geuze H.J., Slot J.W. β-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J. Cell Biol. 1993;121:49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Montesano R., Meda P., Malaisse-Lagae F., Brown D., Perrelet A., Vassalli P. Heterogeneous distribution of filipin-cholesterol complexes across the cisternae of the Golgi apparatus. Proc. Natl. Acad. Sci. USA. 1981;78:293–297. doi: 10.1073/pnas.78.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Glick B.S., Rothman J.E. A new type of coated vesicular carrier that appears not to contain clathrinits possible role in protein transport within the Golgi stack. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Orci L., Malhotra V., Amherdt M., Serafini T., Rothman J.E. Dissection of a single round of vesicular transportsequential intermediates for intercisternal movement in the Golgi stack. Cell. 1989;56:357–368. doi: 10.1016/0092-8674(89)90239-0. [DOI] [PubMed] [Google Scholar]
- Orci L., Schekman R., Perrelet A. Interleaflet clear space is reduced in the membrane of COPI and COPII-coated buds/vesicles. Proc. Natl. Acad. Sci. USA. 1996;93:8968–8970. doi: 10.1073/pnas.93.17.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Stamnes M., Ravazzola M., Amherdt M., Perrelet A., Söllner T.H., Rothman J.E. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Orci L., Tani K., Amherdt M., Ravazzola M., Elazar Z., Rothman J.E. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Pagano R.E. Lipid traffic in eukaryotic cellsmechanisms for intracellular transport and organelle-specific enrichment of lipids. Curr. Opin. Cell Biol. 1990;2:652–663. doi: 10.1016/0955-0674(90)90107-p. [DOI] [PubMed] [Google Scholar]
- Pagano R.E., Sepanski M.A., Martin O.C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cellsinteraction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J. Cell Biol. 1989;109:2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H.R. The dynamic organisation of the secretory pathway. Cell Struct. Funct. 1996;21:413–419. doi: 10.1247/csf.21.413. [DOI] [PubMed] [Google Scholar]
- Pelham H.R. Getting through the Golgi complex. Trends Cell Biol. 1998;8:45–49. doi: 10.1016/s0962-8924(97)01185-9. [DOI] [PubMed] [Google Scholar]
- Pepperkok R., Whitney J.A., Gomez M., Kreis T.E. COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J. Cell Sci. 2000;113:135–144. doi: 10.1242/jcs.113.1.135. [DOI] [PubMed] [Google Scholar]
- Reinhart M.P., Billheimer J.T., Faust J.R., Gaylor J.L. Subcellular localization of the enzymes of cholesterol biosynthesis and metabolism in rat liver. J. Biol. Chem. 1987;262:9649–9655. [PubMed] [Google Scholar]
- Rothman J.E., Wieland F.T. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fleischer S., Yamamoto A. Two dimensional thin layer chromotographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Sandhoff R., Brugger B., Jeckel D., Lehmann W.D., Wieland F.T. Determination of cholesterol at the low picomole level by nano-electrospray ionization tandem mass spectrometry. J. Lipid Res. 1999;40:126–132. [PubMed] [Google Scholar]
- Schmid P.C., Johnson S.B., Schmid H.H. Remodeling of rat hepatocyte phospholipids by selective acyl turnover. J. Biol. Chem. 1991;266:13690–13697. [PubMed] [Google Scholar]
- Schneiter R., Brugger B., Sandhoff R., Zellnig G., Leber A., Lampl M., Athenstaedt K., Hrastnik C., Eder S., Daum G., Paltauf F., Wieland F.T., Kohlwein S.D. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain–based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 1999;146:741–754. doi: 10.1083/jcb.146.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T., Rothman J.E. Purification of Golgi cisternae-derived non-clathrin-coated vesicles. Methods Enzymol. 1992;219:286–299. doi: 10.1016/0076-6879(92)19029-6. [DOI] [PubMed] [Google Scholar]
- Serafini T., Stenbeck G., Brecht A., Lottspeich F., Orci L., Rothman J.E., Wieland F.T. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature. 1991;349:215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Slusarewicz P., Hui N., Warren G. Purification of rat liver Golgi stacks. In: Celis J.E., editor. Cell BiologyA Laboratory Handbook. Academic Press; New York: 1994. pp. 509–516. [Google Scholar]
- Smart E.J., Ying Y., Donzell W.C., Anderson R.G. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J. Biol. Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- Sohn K., Orci L., Ravazzola M., Amherdt M., Bremser M., Lottspeich F., Fiedler K., Helms J.B., Wieland F.T. A major membrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J. Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes M.A., Craighead M.W., Hoe M.H., Lampen N., Geromanos S., Tempst P., Rothman J.E. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc. Natl. Acad. Sci. USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter P.J., Voelker D.R. Lipid transport processes in eukaryotic cells. Biochim. Biophys. Acta. 1994;1213:241–262. doi: 10.1016/0005-2760(94)00073-5. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A., Ying Y., Smart E.J. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J. Biol. Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- Urade R., Hayashi Y., Kito M. Endosomes differ from plasma membranes in the phospholipid molecular species composition. Biochim. Biophys. Acta. 1988;946:151–163. doi: 10.1016/0005-2736(88)90468-3. [DOI] [PubMed] [Google Scholar]
- Urbani L., Simoni R.D. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J. Biol. Chem. 1990;265:1919–1923. [PubMed] [Google Scholar]
- van Helvoort A., van Meer G. Intracellular lipid heterogeneity caused by topology of synthesis and specificity in transport. Examplesphingolipids. FEBS Lett. 1995;369:18–21. doi: 10.1016/0014-5793(95)00616-h. [DOI] [PubMed] [Google Scholar]
- van Meer G. Lipid traffic in animal cells. Annu. Rev. Cell Biol. 1989;5:247–275. doi: 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]
- van Meer G. Transport and sorting of membrane lipids. Curr. Opin. Cell Biol. 1993;5:661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- van Meer G. Lipids of the Golgi membrane. Trends Cell Biol. 1998;8:29–33. doi: 10.1016/s0962-8924(97)01196-3. [DOI] [PubMed] [Google Scholar]
- Volchuk A., Amherdt M., Ravazzola M., Brügger B., Rivera V., Clackson T., Perrelet A., Söllner T.H., Rothman J.E., Orci L. Mega-vesicles implicated in the rapid transport of intra-cisternal aggregates across the Golgi stack. Cell. 2000;102:335–348. doi: 10.1016/s0092-8674(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Warnock D.E., Roberts C., Lutz M.S., Blackburn W.A., Young W.W., Jr., Baenziger J.U. Determination of plasma membrane lipid mass and composition in cultured Chinese hamster ovary cells using high gradient magnetic affinity chromatography. J. Biol. Chem. 1993;268:10145–10153. [PubMed] [Google Scholar]
- Warren G., Malhotra V. The organisation of the Golgi apparatus. Curr. Opin. Cell Biol. 1998;10:493–498. doi: 10.1016/s0955-0674(98)80064-1. [DOI] [PubMed] [Google Scholar]