Abstract
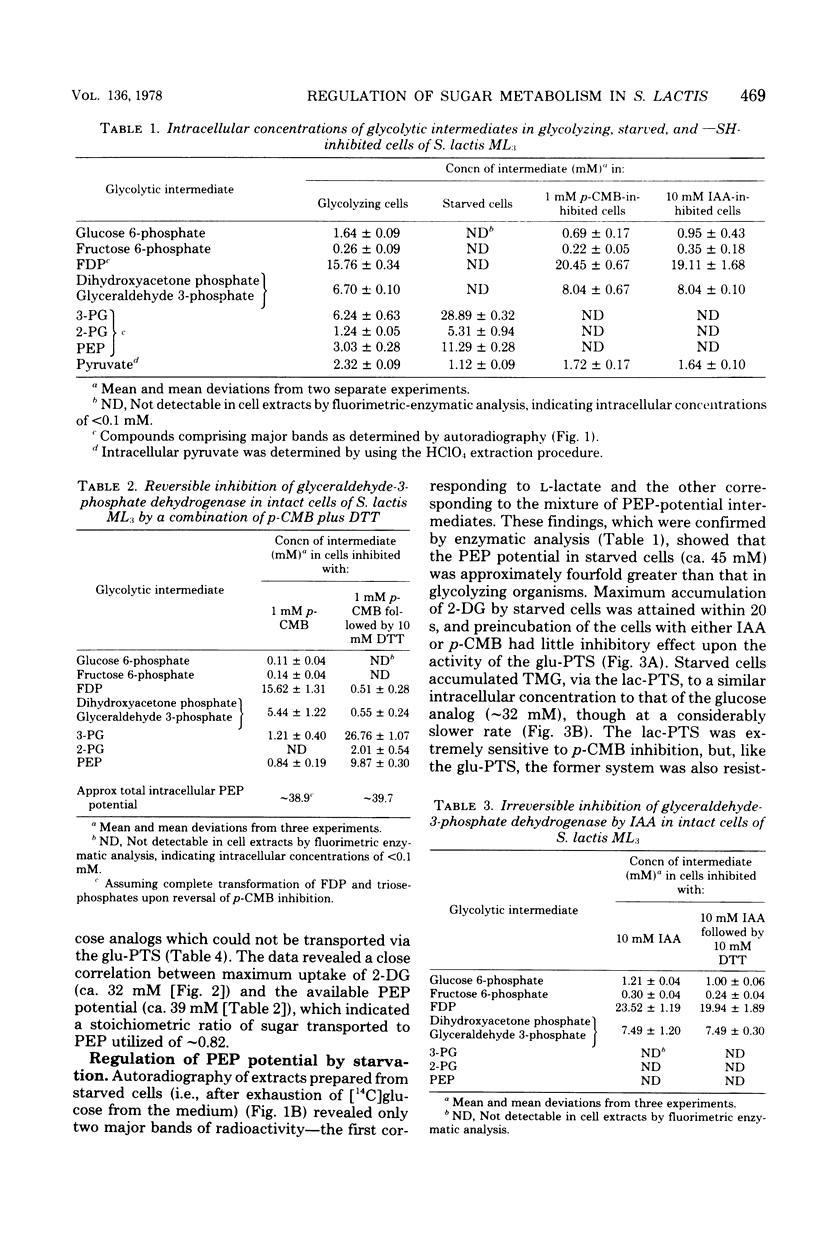
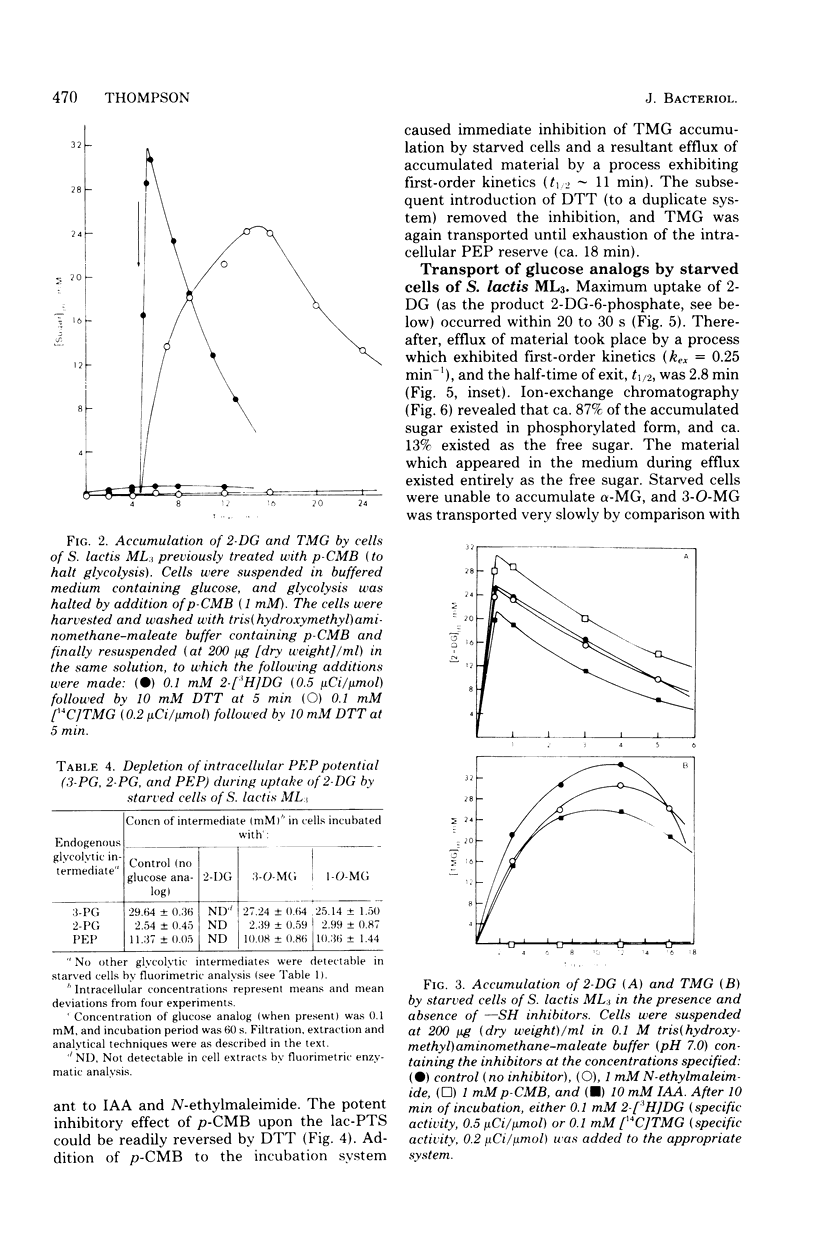
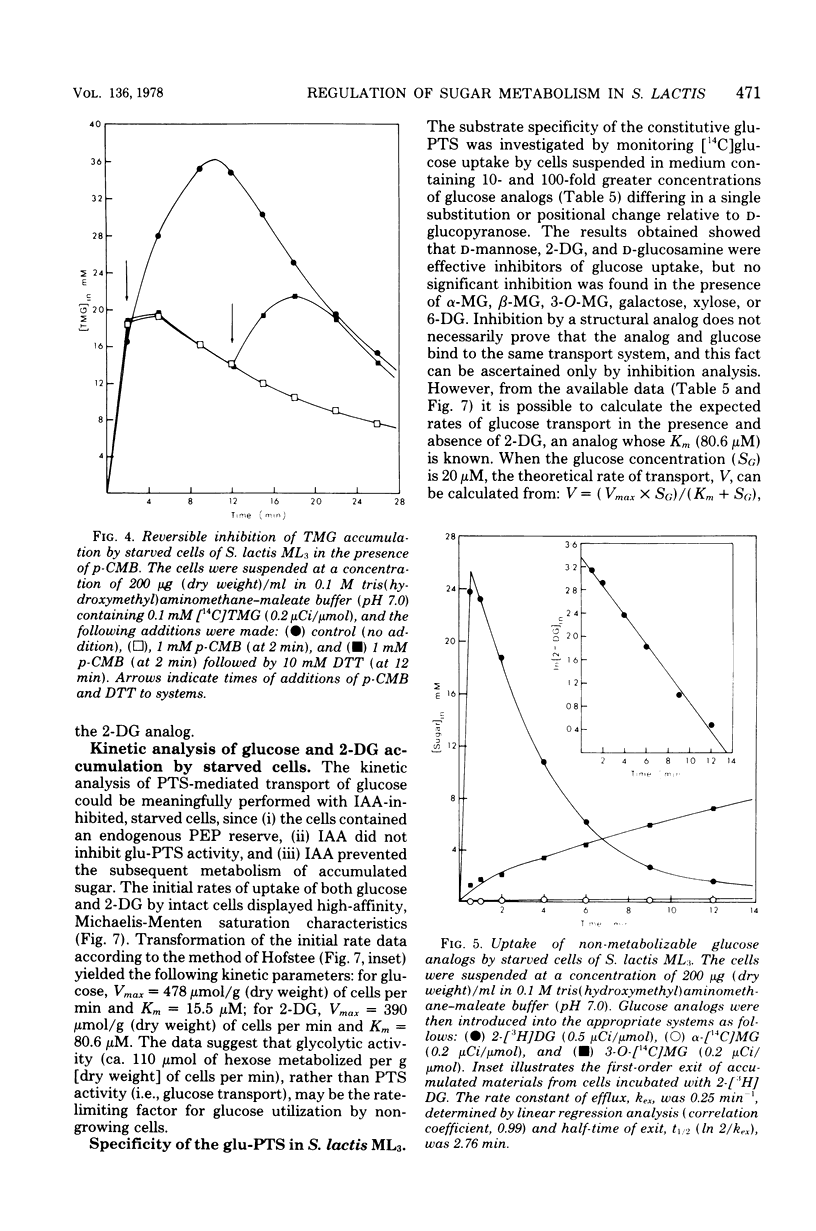
Two novel procedures have been used to regulate, in vivo, the formation of phosphoenolpyruvate (PEP) from glycolysis in Streptococcus lactis ML3. In the first procedure, glucose metabolism was specifically inhibited by p-chloromercuribenzoate. Autoradiographic and enzymatic analyses showed that the cells contained glucose 6-phosphate, fructose 6-phosphate, fructose-1,6-diphosphate, and triose phosphates.Dithiothreitol reversed the p-chloromercuribenzoate inhibition, and these intermediates were rapidly and quantitatively transformed into 3- and 2-phosphoglycerates plus PEP. The three intermediates were not further metabolized and constituted the intracellular PEP potential. The second procedure simply involved starvation of the organisms. The starved cells were devoid of glucose 6-phosphate, fructose 6-phosphate, fructose- 1,6-diphosphate, and triose phosphates but contained high levels of 3- and 2-phosphoglycerates and PEP (ca. 40 mM in total). The capacity to regulate PEP formation in vivo permitted the characterization of glucose and lactose phosphotransferase systems in physiologically intact cells. Evidence has been obtained for "feed forward" activation of pyruvate kinase in vivo by phosphorylated intermediates formed before the glyceraldehyde-3-phosphate dehydrogenase reaction in the glycolytic sequence. The data suggest that pyruvate kinase (an allosteric enzyme) plays a key role in the regulation of glycolysis and phosphotransferase system functions in S. lactis ML3.
Full text
PDF



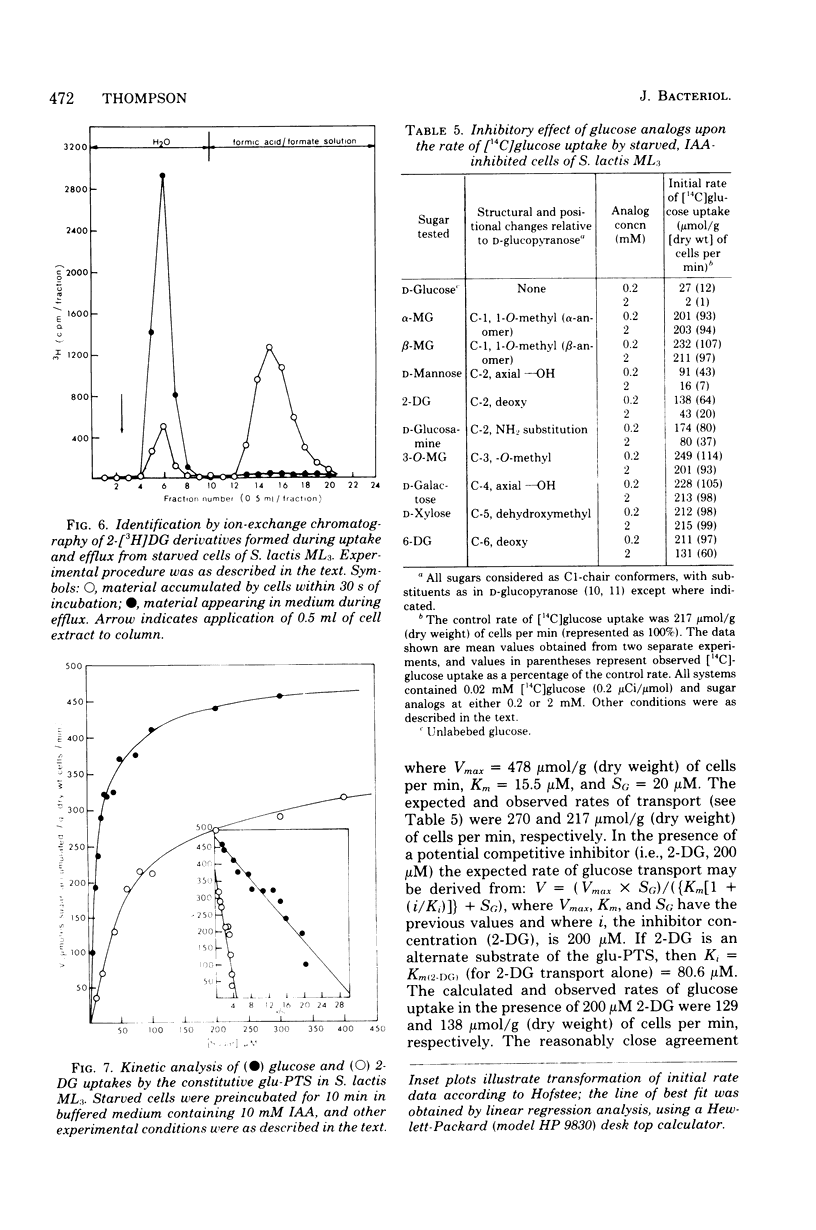

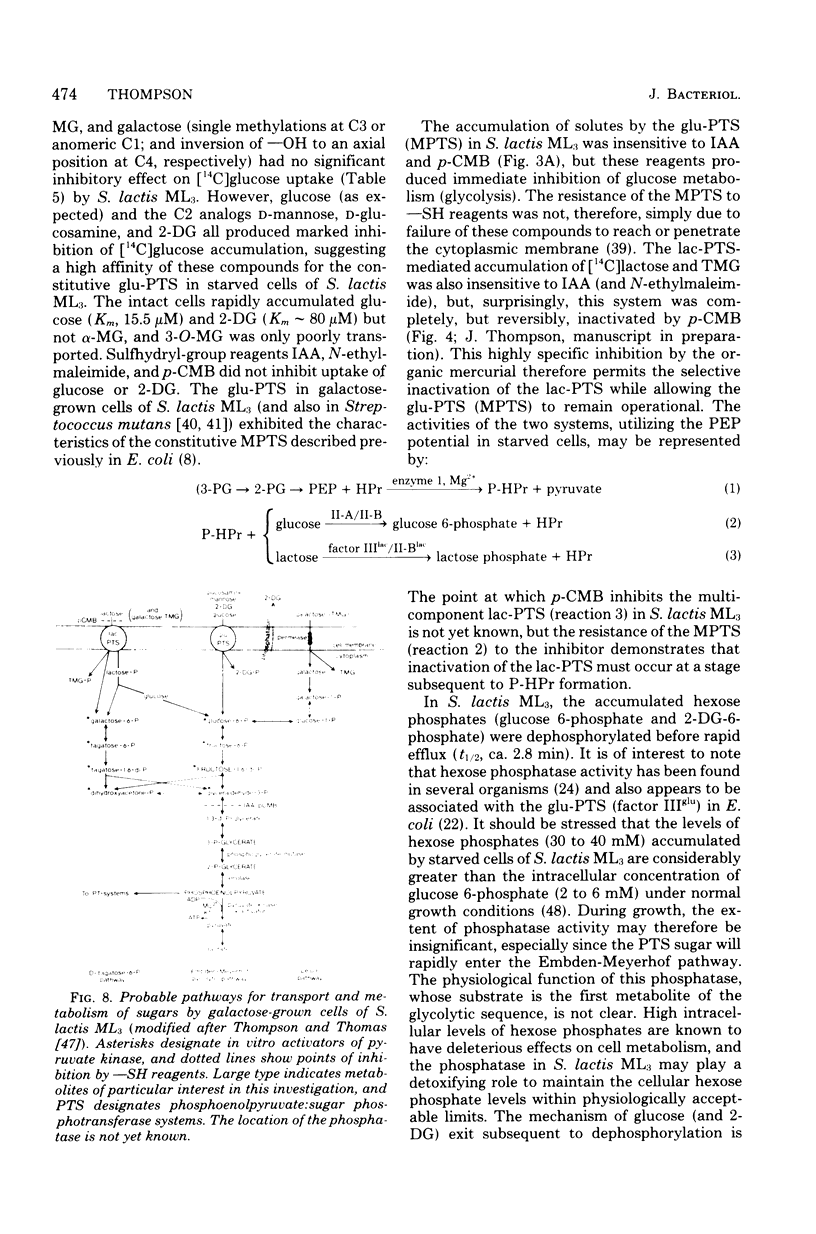


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P., Razin S. Distribution of a phosphoenolypyruvate-dependent sugar phosphotransferase system in mycoplasms. J Bacteriol. 1973 Jan;113(1):212–217. doi: 10.1128/jb.113.1.212-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P. Relationship between sugar structure and competition for the sugar transport system in Bakers' yeast. J Bacteriol. 1968 Feb;95(2):603–611. doi: 10.1128/jb.95.2.603-611.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B., Holms W. H. Control of the sequential utilization of glucose and fructose by Escherichia coli. J Gen Microbiol. 1976 Aug;96(2):191–201. doi: 10.1099/00221287-95-2-191. [DOI] [PubMed] [Google Scholar]
- Collins L. B., Thomas T. D. Pyruvate kinase of Streptococcus lactis. J Bacteriol. 1974 Oct;120(1):52–58. doi: 10.1128/jb.120.1.52-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conyers R. A., Newsholme E. A., Brand K. A thin-layer-chromatographic method for the separation of sugar phosphates. Biochem Soc Trans. 1976;4(6):1040–1042. doi: 10.1042/bst0041040. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Purification and properties of pyruvate kinase from Streptococcus lactis. Biochim Biophys Acta. 1976 Jun 7;438(1):90–101. doi: 10.1016/0005-2744(76)90225-4. [DOI] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachelin G. A new assay of the phosphotransferase system in Escherichia coli. Biochem Biophys Res Commun. 1969 Feb 21;34(4):382–387. doi: 10.1016/0006-291x(69)90392-1. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Ghosh D. Probable role of a membrane-bound phosphoenolpyruvate-hexose phosphotransferase system of Escherichia coli in the permeation of sugars. Indian J Biochem. 1968 Jun;5(2):49–52. [PubMed] [Google Scholar]
- Haguenauer-Tsapis R., Kepes A. Changes in accessibility of the membrane bound transport enzyme glucose phosphotransferase of E. coli to protein group reagents in presence of substrate or absence of energy source. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1335–1341. doi: 10.1016/0006-291x(73)91133-9. [DOI] [PubMed] [Google Scholar]
- Haguenauer-Tsapis R., Kepes A. Unmasking of an essential thiol during function of the membrane bound enzyme II of the phosphoenolpyruvate glucose phosphotransferase system of Escherichia coli. Biochim Biophys Acta. 1977 Feb 14;465(1):118–130. doi: 10.1016/0005-2736(77)90360-1. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M. H., Maitra P. K. Properties of Escherichia coli mutants deficient in enzymes of glycolysis. J Bacteriol. 1977 Nov;132(2):398–410. doi: 10.1128/jb.132.2.398-410.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Correlation between hexose transport and phosphotransferase activity in Escherichia coli. Biochem J. 1972 Mar;126(5):1241–1243. doi: 10.1042/bj1261241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Inducible phosphoenolpyruvate-dependent hexose phosphotransferase activities in Escherichia coli. Biochem J. 1972 Aug;128(5):1339–1344. doi: 10.1042/bj1281339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W. Molecular interactions in the bacterial phosphoenolpyruvate-phosphotransferase system (PTS). J Supramol Struct. 1974;2(5-6):695–814. doi: 10.1002/jss.400020514. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. THE RELATIONSHIPS BETWEEN SUBSTRATES AND ENZYMES OF GLYCOLYSIS IN BRAIN. J Biol Chem. 1964 Jan;239:31–42. [PubMed] [Google Scholar]
- Lee Y. P., Sowokinos J. R. Sugar phosphate phosphohydrolase. I. Substrate specificity, intracellular localization, and purification from Neisseria meningitidis. J Biol Chem. 1967 May 10;242(9):2264–2271. [PubMed] [Google Scholar]
- MAITRA P. K., ESTABROOK R. W. A FLUOROMETRIC METHOD FOR THE ENZYMIC DETERMINATION OF GLYCOLYTIC INTERMEDIATES. Anal Biochem. 1964 Apr;7:472–484. doi: 10.1016/0003-2697(64)90156-3. [DOI] [PubMed] [Google Scholar]
- MIZUSHIMA S., KITAHARA K. QUANTITATIVE STUDIES ON GLYCOLYTIC ENZYMES IN LACTOBACILLUS PLANTARUM. II. INTRACELLULAR CONCENTRATIONS OF GLYCOLYTIC INTERMEDIATES IN GLUCOSE-METABOLIZING WASHED CELLS. J Bacteriol. 1964 Jun;87:1429–1435. doi: 10.1128/jb.87.6.1429-1435.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith S. A., Romano A. H. Uptake and phosphorylation of 2-deoxy-D-glucose by wild type and respiration-deficient bakers' yeast. Biochim Biophys Acta. 1977 May 26;497(3):745–759. doi: 10.1016/0304-4165(77)90295-1. [DOI] [PubMed] [Google Scholar]
- Négrel R., Ailhaud G., Mutaftschiev S. Comparative inhibition studies of the phosphotransferase and glycerophosphate acylation systems in membrane vesicles of Escherichia coli. Biochim Biophys Acta. 1973 Feb 16;291(3):635–649. doi: 10.1016/0005-2736(73)90469-0. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F. Glucose transport in Streptococcus mutans: preparation of cytoplasmic membranes and characteristics of phosphotransferase activity. J Dent Res. 1975 Mar-Apr;54(2):330–338. [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Thomas T. D. Activator specificity of pyruvate kinase from lactic streptococci. J Bacteriol. 1976 Mar;125(3):1240–1242. doi: 10.1128/jb.125.3.1240-1242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Turner K. W., Thomas T. D. Catabolite inhibition and sequential metabolism of sugars by Streptococcus lactis. J Bacteriol. 1978 Mar;133(3):1163–1174. doi: 10.1128/jb.133.3.1163-1174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]