Abstract
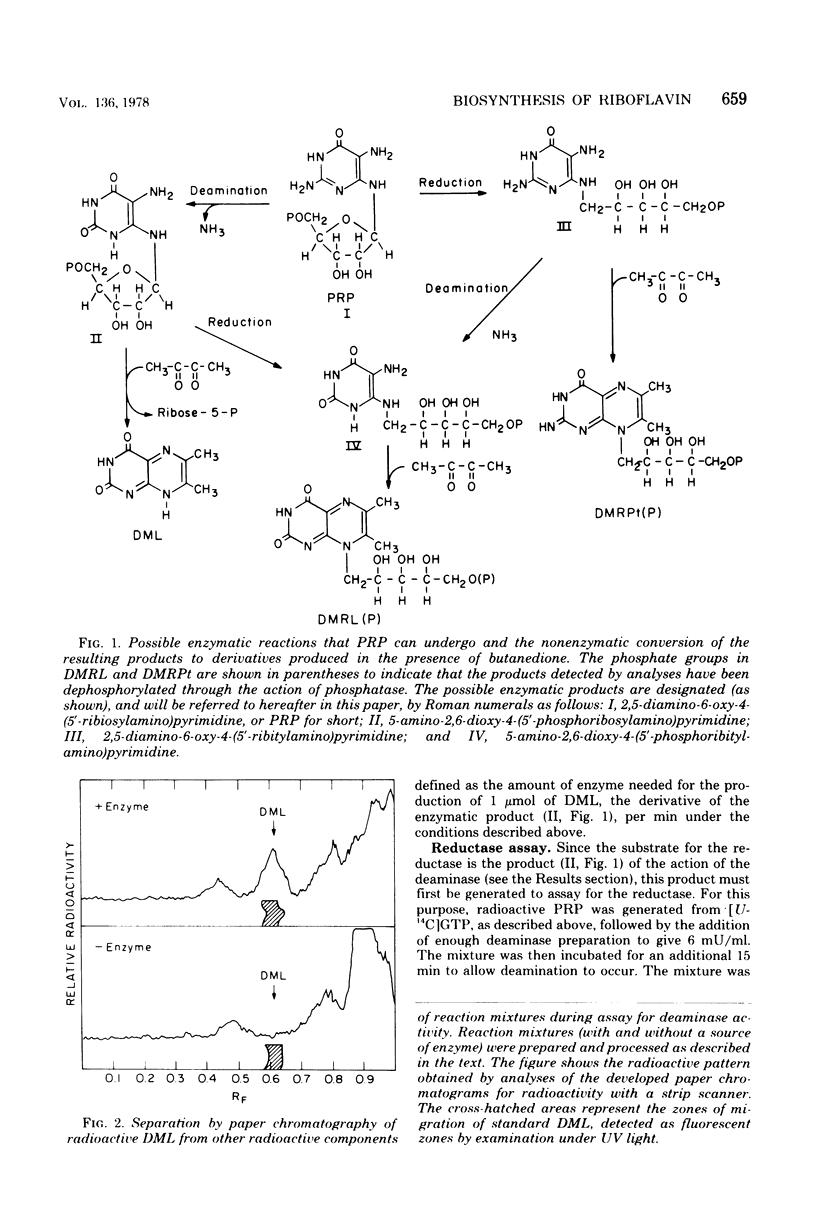
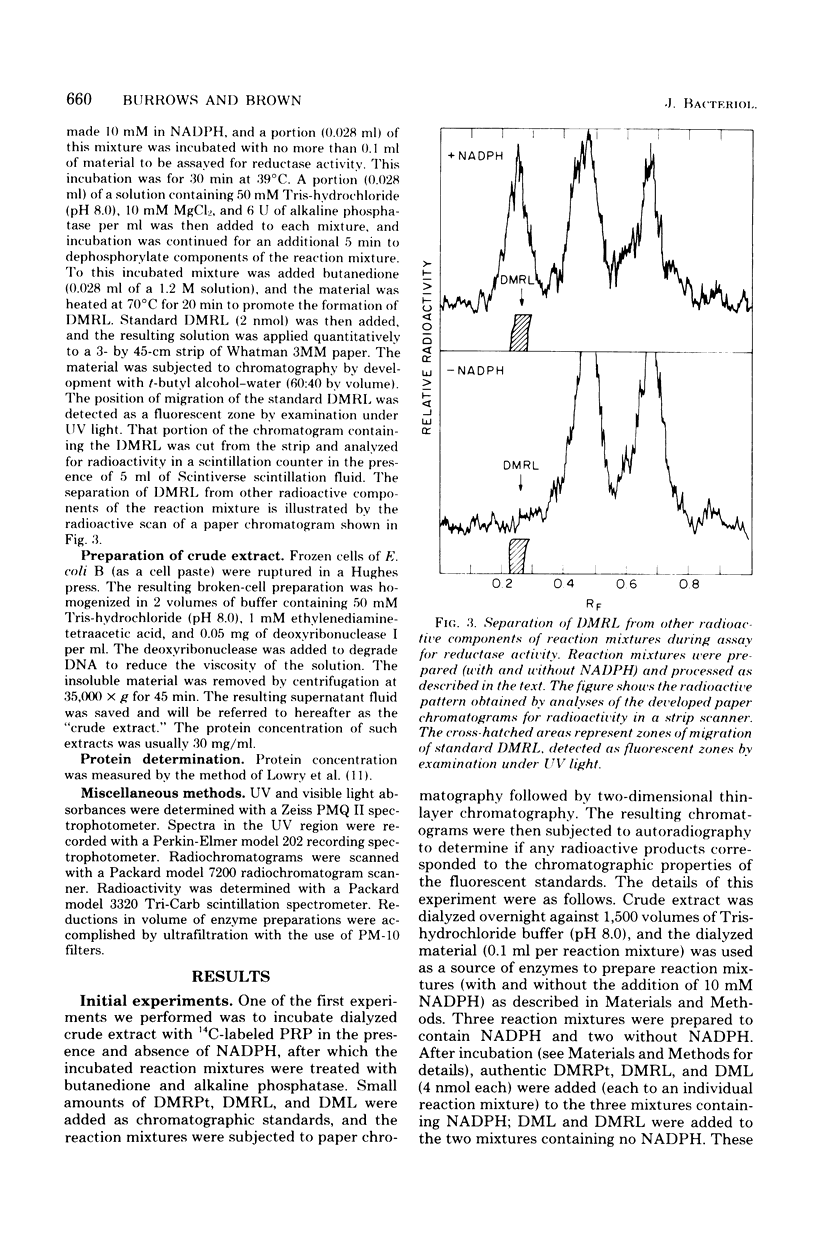
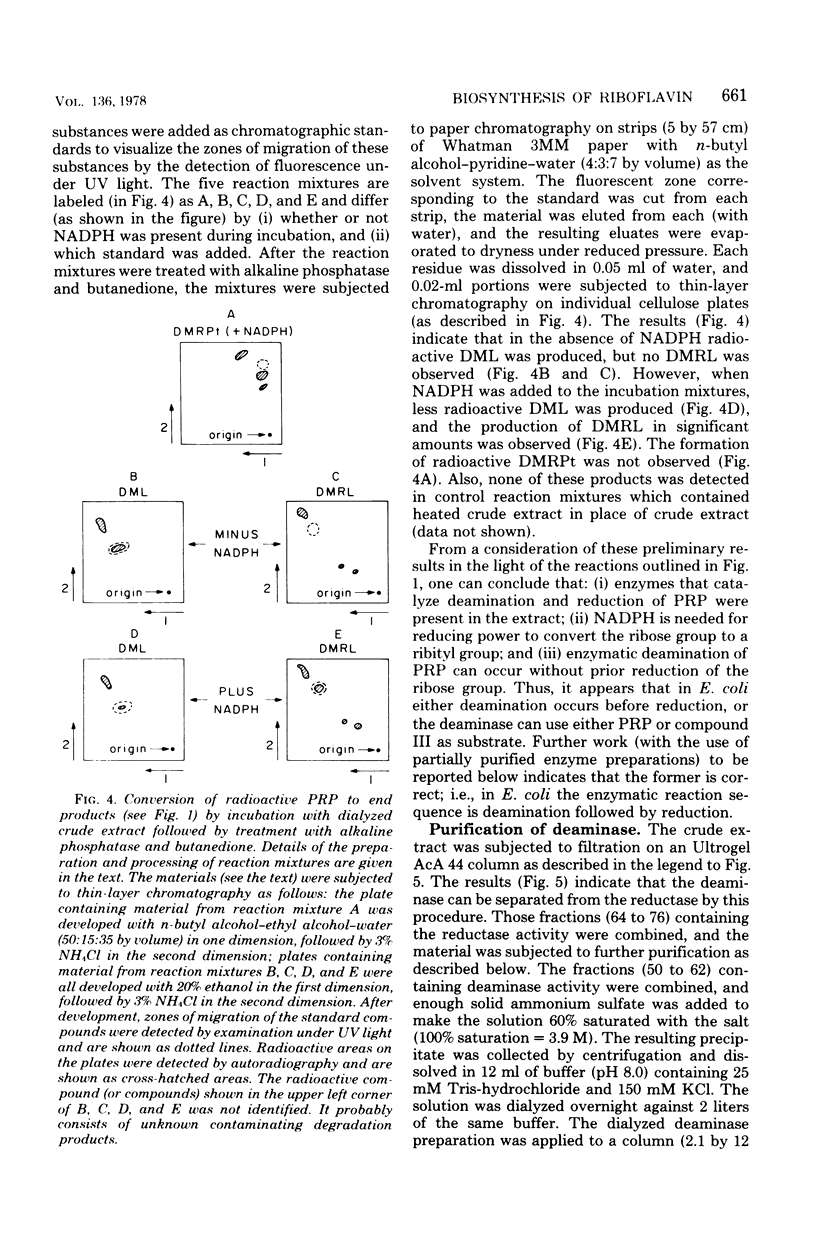
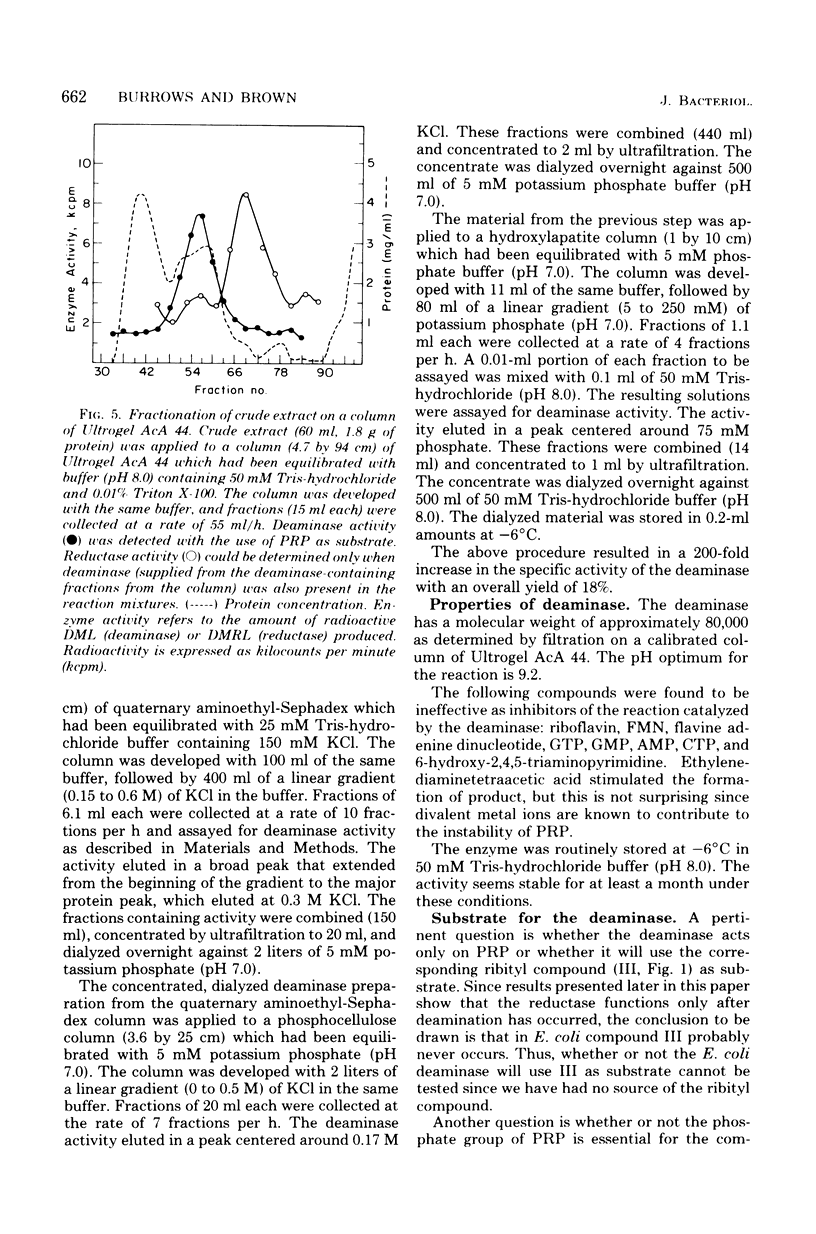
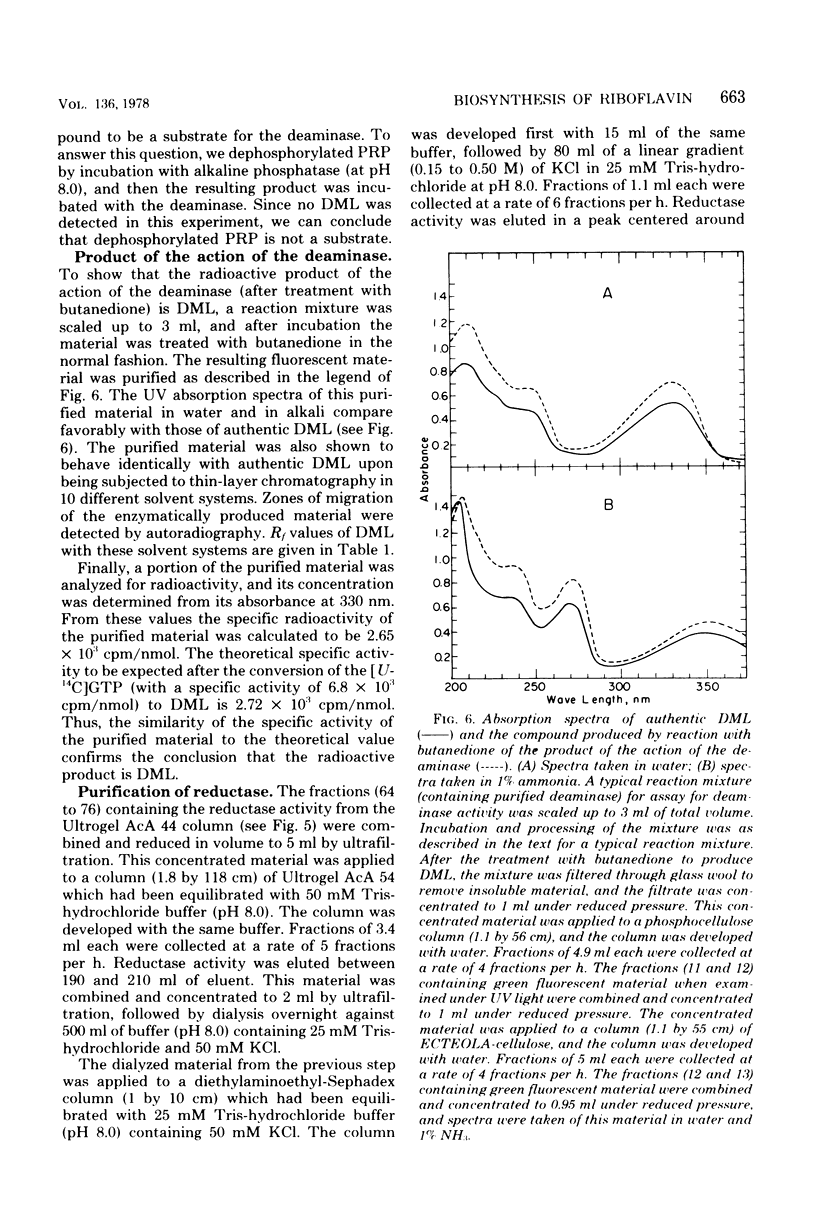
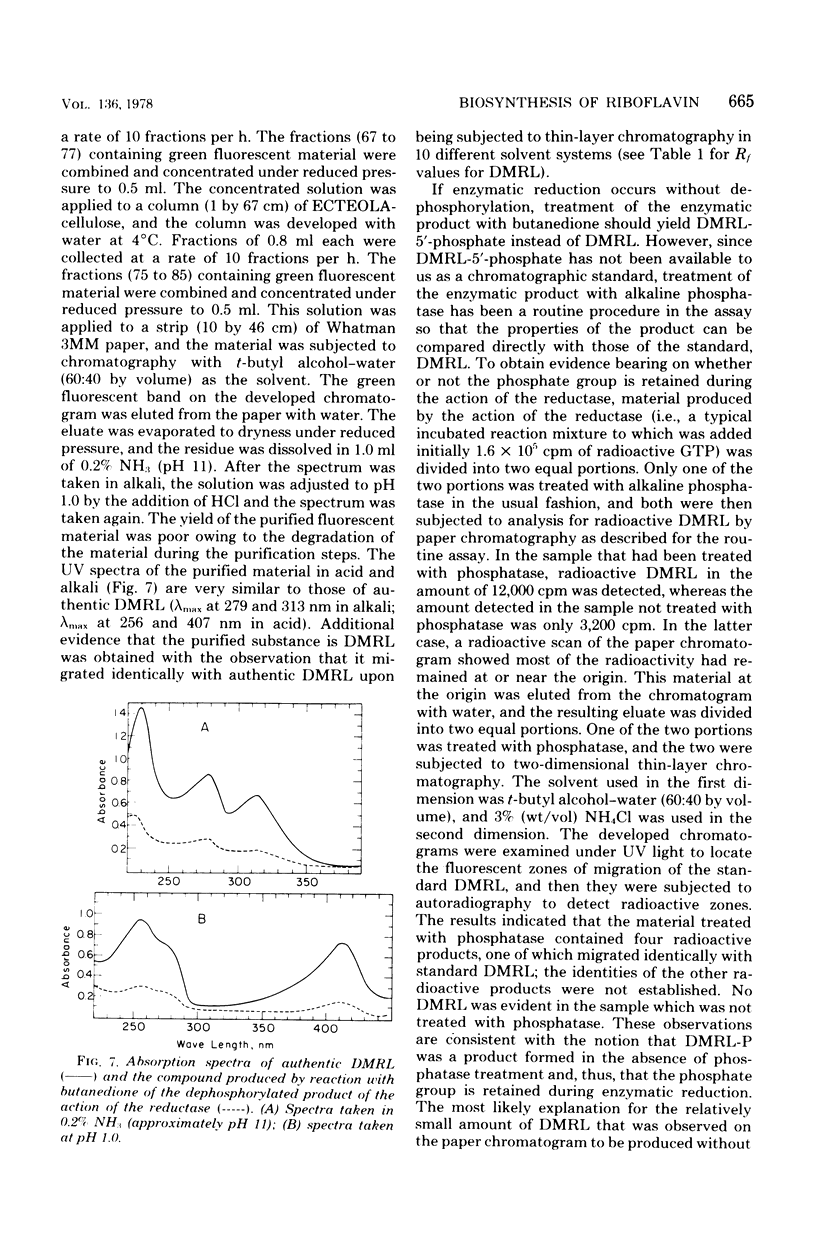
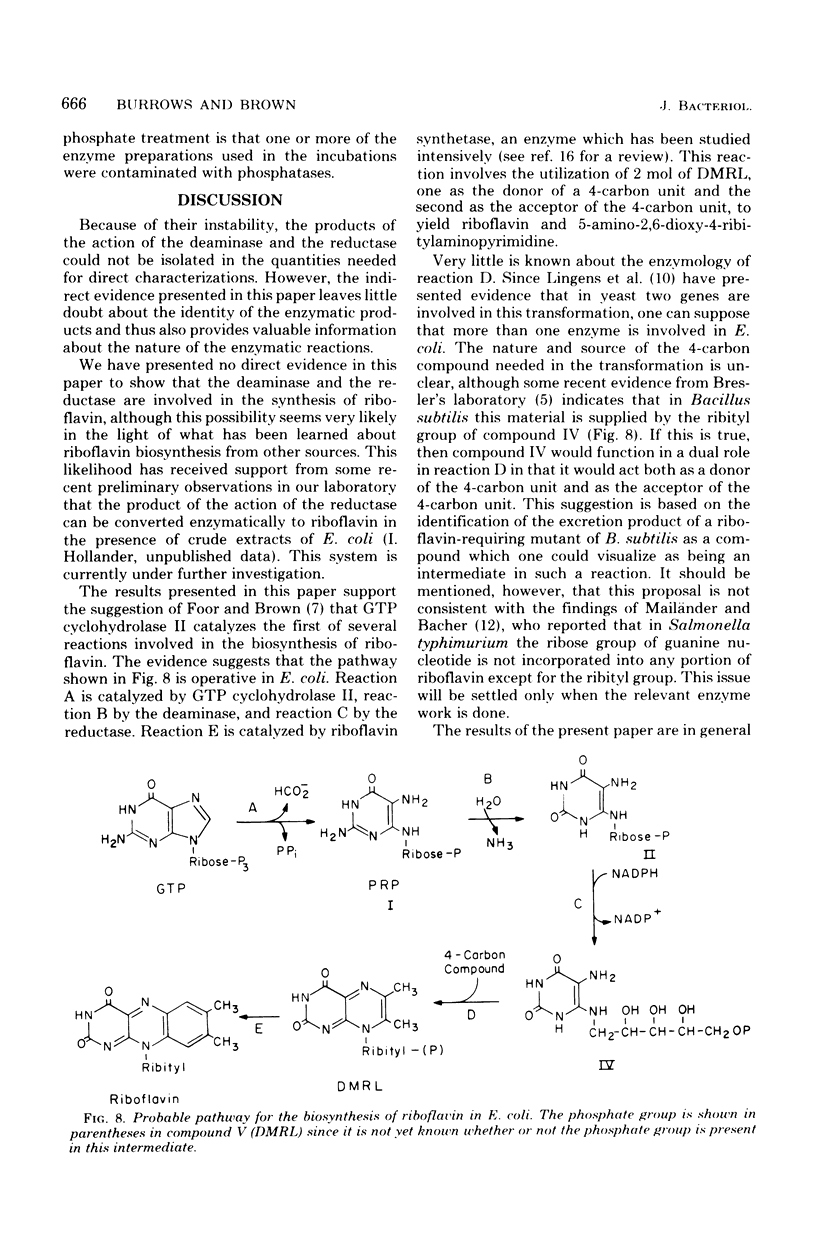
Two enzymes have been partially purified from extracts of Escherchia coli B which together catalyze the conversion of the product of the action of GTP cyclohydrolase II, 2,5-diamino-6-oxy-4-(5'-phosphoribosylamine)pyrimidine, to 5-amino-2,6-dioxy-4-(5'-phosphoribitylamine)pyrimidine. These two compounds are currently thought to be intermediates in the biosynthesis of riboflavin. The enzymatic conversion occurs in two steps. The product of the action of GTP cyclohydrolase II first undergoes hydrolytic deamination at carbon 2 of the ring, followed by reduction of the ribosylamino group to a ribitylamino group. The enzyme which catalyzes the first step, herein called the "deaminase," has been purified 200-fold. The activity was assayed by detecting the conversion of the product of the reaction catalyzed by GTP cyclohydrolase II to a compound which reacts with butanedione to form 6,7-dimethyllumazine. The enzyme has a molecular weight of approximately 80,000 and a pH optimum of 9.1. The dephosphorylated form of the substrate is not deaminated in the presence of the enzyme. The assay for the enzyme which catalyzes the second step, referred to here as the "reductase," involves the detection of the conversion of the product of the deaminase-catalyzed reaction to a compound which, after treatment with alkaline phosphatase, reacts with butanedione to form 6,7-dimethyl-8-ribityllumazine. The reductase has a molecular weight of approximately 40,000 and a pH optimum of 7.5. Like the deaminase, the reductase does not act on the dephosphorylated form of its substrate. Reduced nicotinamide adenine dinucleotide phosphate is required as a cofactor; reduced nicotinamide adenine dinucleotide can be used about 30% as well as the phosphate form. The activity of neither enzyme is inhibited by riboflavin, FMN, or flavine adenine dinucleotide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacher A., Lingens F. Biosynthesis of riboflavin. Formation of 2,5-diamino-6-hydroxy-4-(1'-D-ribitylamino)pyrimidine in a riboflavin auxotroph. J Biol Chem. 1970 Sep 25;245(18):4647–4652. [PubMed] [Google Scholar]
- Bacher A., Mailänder B. Biosynthesis of riboflavin. The structure of the purine precursor. J Biol Chem. 1973 Sep 10;248(17):6227–6231. [PubMed] [Google Scholar]
- Baugh C. M., Krumdieck C. L. Biosynthesis of riboflavine in Corynebacterium species: the purine precursor. J Bacteriol. 1969 Jun;98(3):1114–1119. doi: 10.1128/jb.98.3.1114-1119.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foor F., Brown G. M. Purification and properties of guanosine triphosphate cyclohydrolase II from Escherichia coli. J Biol Chem. 1975 May 10;250(9):3545–3551. [PubMed] [Google Scholar]
- Harvey R. A., Plaut G. W. Riboflavin synthetase from yeast. Properties of complexes of the enzyme with lumazine derivatives and riboflavin. J Biol Chem. 1966 May 10;241(9):2120–2136. [PubMed] [Google Scholar]
- Harzer G., Rokos H., Otto M. K., Bacher A., Ghisla S. Biosynthesis of riboflavin. 6,7-Dimethyl-8-ribityllumazine 5'-phosphate is not a substrate for riboflavin synthase. Biochim Biophys Acta. 1978 Apr 19;540(1):48–54. doi: 10.1016/0304-4165(78)90433-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lingens F., Oltmanns O., Bacher A. Uber Zwischenprodukte der Riboflavin-Biosynthese bei Saccharomyces cerevisiae. Z Naturforsch B. 1967 Jul;22(7):755–758. [PubMed] [Google Scholar]
- MALEY G. F., PLAUT G. W. The isolation, synthesis, and metabolic properties of 6, 7-dimethyl-8-ribityllumazine. J Biol Chem. 1959 Mar;234(3):641–647. [PubMed] [Google Scholar]
- Mailänder B., Bacher A. Biosynthesis of riboflavin. Structure of the purine precursor and origin of the ribityl side chain. J Biol Chem. 1976 Jun 25;251(12):3623–3628. [PubMed] [Google Scholar]
- Oltmanns O., Bacher A. Biosynthesis of riboflavine in Saccharomyces cerevisiae: the role of genes rib 1 and rib 7 . J Bacteriol. 1972 Jun;110(3):818–822. doi: 10.1128/jb.110.3.818-822.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut G. W., Smith C. M., Alworth W. L. Biosynthesis of water-soluble vitamins. Annu Rev Biochem. 1974;43(0):899–922. doi: 10.1146/annurev.bi.43.070174.004343. [DOI] [PubMed] [Google Scholar]


