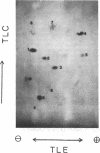
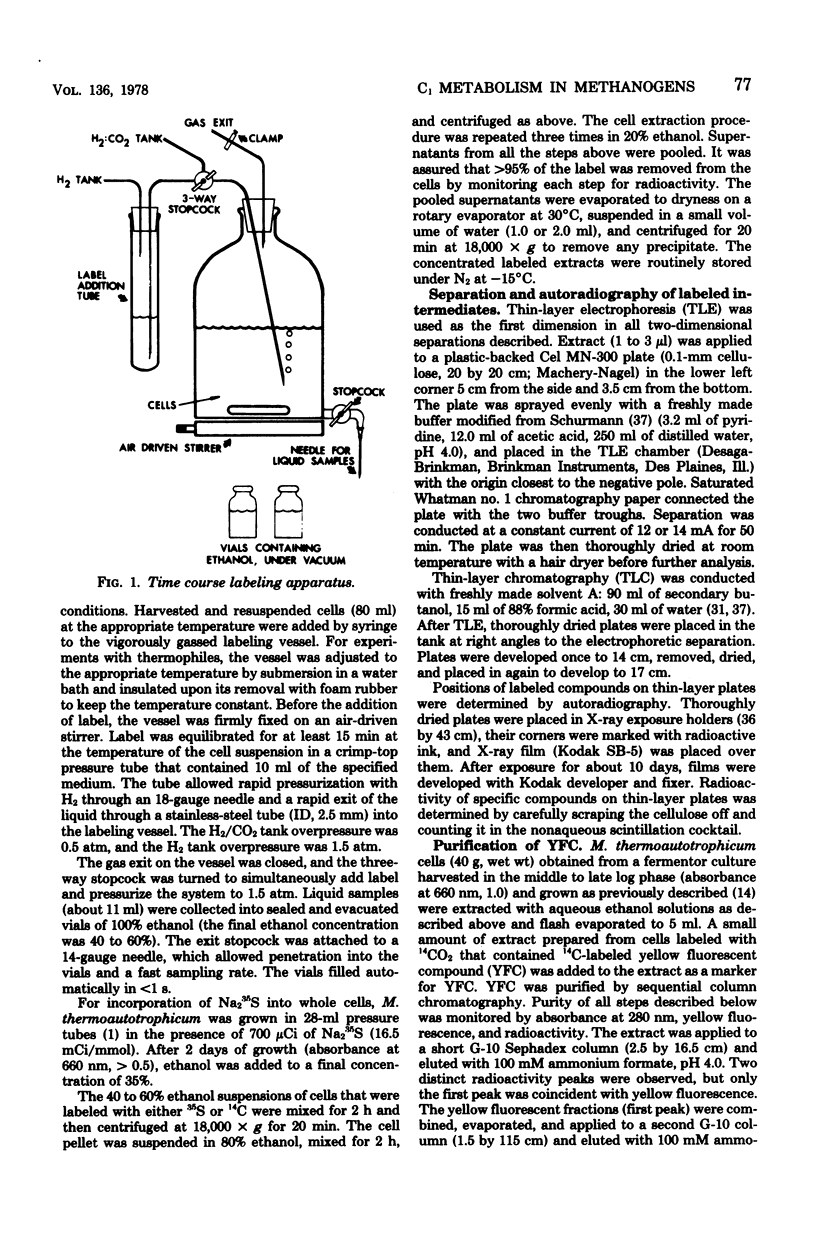
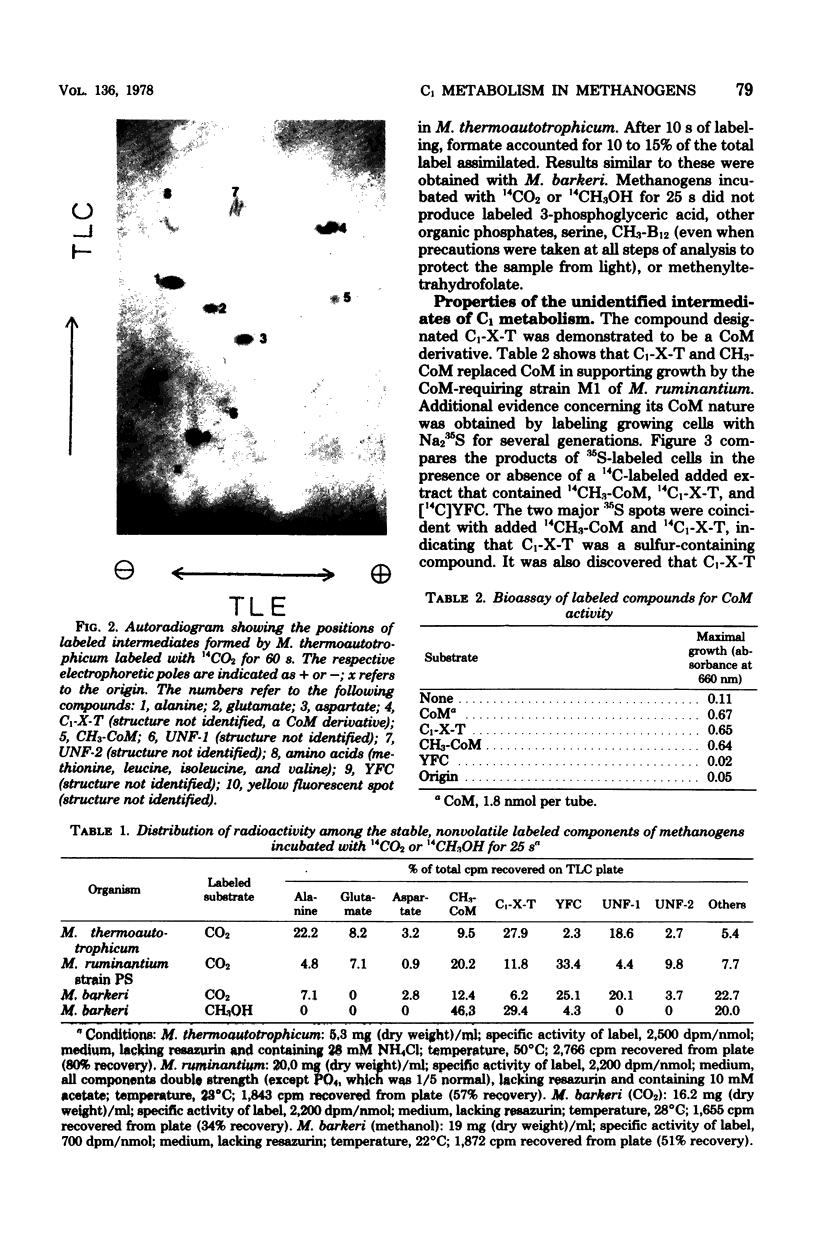
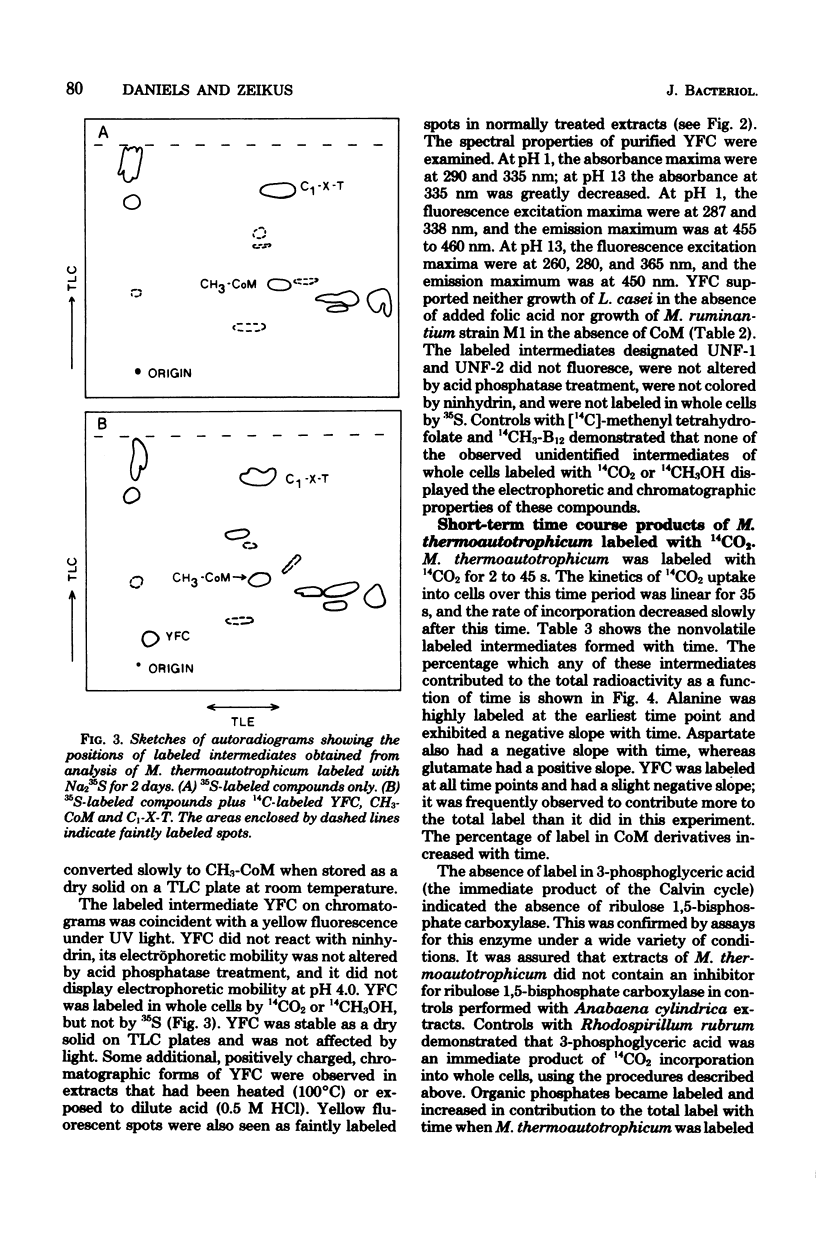
Abstract
Methanobacterium thermoautotrophicum, M. ruminantium, and Methanosarcina barkeri were labeled with 14CO2 (14CO2 + H14CO3- + 14CO32-) for from 2 to 45 s. Radioactivity was recovered in coenzyme M derivatives, alanine, aspartate, glutamate, and several unidentified compounds. The properties of one important structurally unidentified intermediate (yellow fluorescent compound) displayed UV absorbance maxima at pH 1 of 290 and 335 nm, no absorbance in the visible region, and a fluorescence maximum at 460 nm. Label did not appear in organic phosphates until after 1 min. 14CH3OH was converted by M. barkeri primarily into coenzyme M derivatives at 25 s. [2-14C]acetate was assimilated by M. thermoautotrophicum mainly into alanine and succinate during 2 to 240 s, but not into coenzyme M derivatives or yellow fluorescent compound. Cell-free extracts of M. thermoautotrophicum lacked ribulose 1,5-bisphosphate carboxylase activity. The data indicated the absence of the Calvin, serine, and hexulose phosphate paths of C1 assimilation in the methanogens examined and indicated that pyruvate was an early intermediate product of net CO2 fixation. The in vivo importance of coenzyme M derivatives in methanogenesis was demonstrated.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A. Biochemical functions of corrinoid compounds. The sixth Hopkins memorial lecture. Biochem J. 1967 Oct;105(1):1–15. doi: 10.1042/bj1050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A. Corrinoid-dependent enzymic reactions. Annu Rev Biochem. 1972;41:55–90. doi: 10.1146/annurev.bi.41.070172.000415. [DOI] [PubMed] [Google Scholar]
- Blaylock B. A. Cobamide-dependent methanol-cyanocob(I)alamin methyltransferase of Methanosarcina barkeri. Arch Biochem Biophys. 1968 Mar 20;124(1):314–324. doi: 10.1016/0003-9861(68)90333-0. [DOI] [PubMed] [Google Scholar]
- Blaylock B. A., Stadtman T. C. Methane biosynthesis by Methanosarcina barkeri. Properties of the soluble enzyme system. Arch Biochem Biophys. 1966 Sep 26;116(1):138–152. doi: 10.1016/0003-9861(66)90022-1. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Shanmugam K. T. Role of the reductive carboxylic acid cycle in a photosynthetic bacterium lacking ribulose I,5-diphosphate carboxylase. Biochim Biophys Acta. 1972;283(1):136–145. doi: 10.1016/0005-2728(72)90105-3. [DOI] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. Improved culture flask for obligate anaerobes. Appl Microbiol. 1975 May;29(5):710–711. doi: 10.1128/am.29.5.710-711.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T., McBride B. C. New method for the isolation and identification of methanogenic bacteria. Appl Microbiol. 1975 Apr;29(4):540–545. doi: 10.1128/am.29.4.540-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Buchanan B. B., Arnon D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):928–934. doi: 10.1073/pnas.55.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Stimulation of CO2 reduction to methane by methylcoenzyme M in extracts Methanobacterium. Biochem Biophys Res Commun. 1977 Jun 6;76(3):790–795. doi: 10.1016/0006-291x(77)91570-4. [DOI] [PubMed] [Google Scholar]
- JOHNSON P. A., QUAYLE J. R. MICROBIAL GROWTH ON C1 COMPOUNDS. SYNTHESIS OF CELL CONSTITUENTS BY METHANE- AND METHANOL-GROWN PSEUDOMONAS METHANICA. Biochem J. 1965 Jun;95:859–867. doi: 10.1042/bj0950859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- McFadden B. A. Autotrophic CO2 assimilation and the evolution of ribulose diphosphate carboxylase. Bacteriol Rev. 1973 Sep;37(3):289–319. doi: 10.1128/br.37.3.289-319.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Denend A. R. Ribulose diphosphate carboxylase from autotrophic microorganisms. J Bacteriol. 1972 May;110(2):633–642. doi: 10.1128/jb.110.2.633-642.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard P. Two-way separation of carboxylic acids by thin layer electrophoresis and chromatography. J Chromatogr. 1967 Sep;30(1):240–243. doi: 10.1016/s0021-9673(00)84145-x. [DOI] [PubMed] [Google Scholar]
- Schürmann P. Separation of phosphate esters and algal extracts by thin-layer electrophoresis and chromatography. J Chromatogr. 1969 Feb 25;39(4):507–509. [PubMed] [Google Scholar]
- Stadtman T. C. Methane fermentation. Annu Rev Microbiol. 1967;21:121–142. doi: 10.1146/annurev.mi.21.100167.001005. [DOI] [PubMed] [Google Scholar]
- Taylor C. D., McBride B. C., Wolfe R. S., Bryant M. P. Coenzyme M, essential for growth of a rumen strain of Methanobacterium ruminantium. J Bacteriol. 1974 Nov;120(2):974–975. doi: 10.1128/jb.120.2.974-975.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Tokumitsu Y., Michio U. I. Separation and determination of 14C-labelled intermediates of the citric acid cycle and related compounds. Anal Biochem. 1974 May;59(1):110–121. doi: 10.1016/0003-2697(74)90015-3. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., WOLFE R. S. ATP-DEPENDENT FORMATION OF METHANE FROMMETHYLCOBALAMIN BY EXTRACTS OF METHANOBACILLUS OMELIANSKII. Biochem Biophys Res Commun. 1963 Aug 20;12:464–468. doi: 10.1016/0006-291x(63)90316-4. [DOI] [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]