Abstract
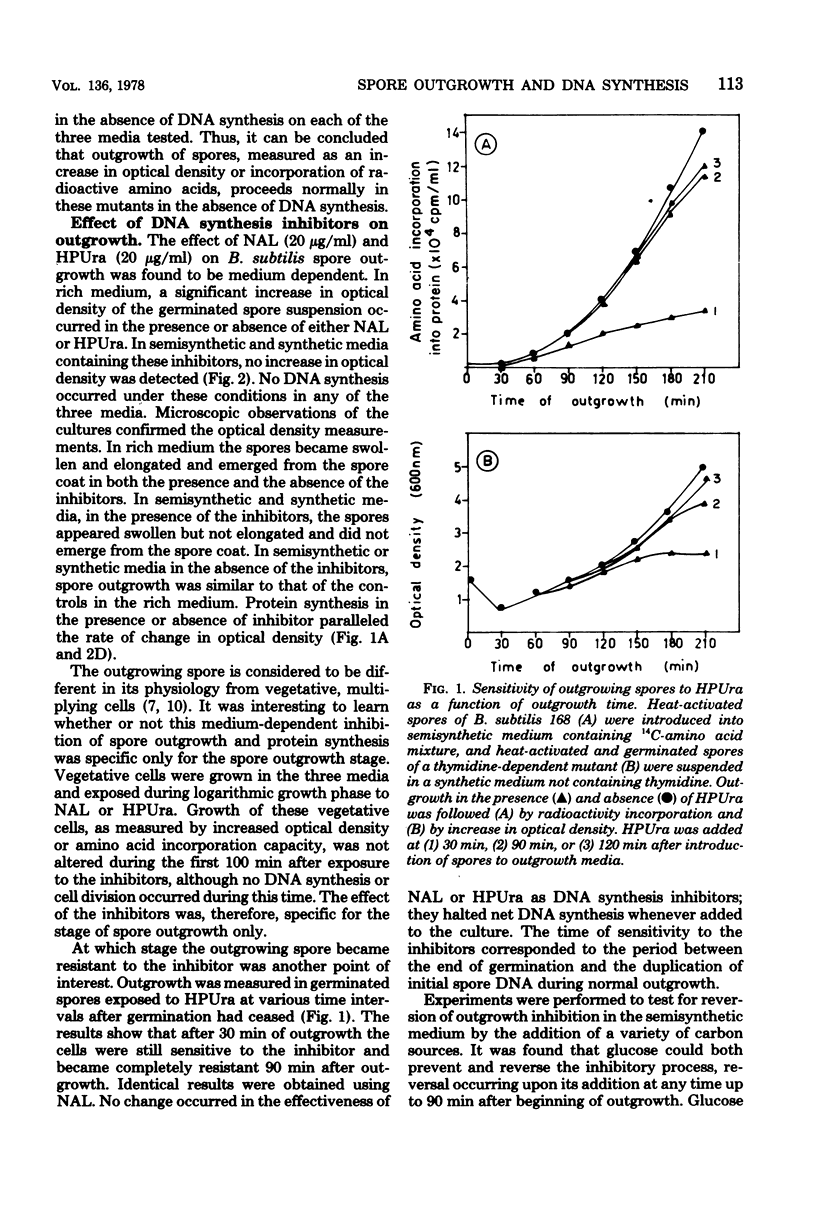
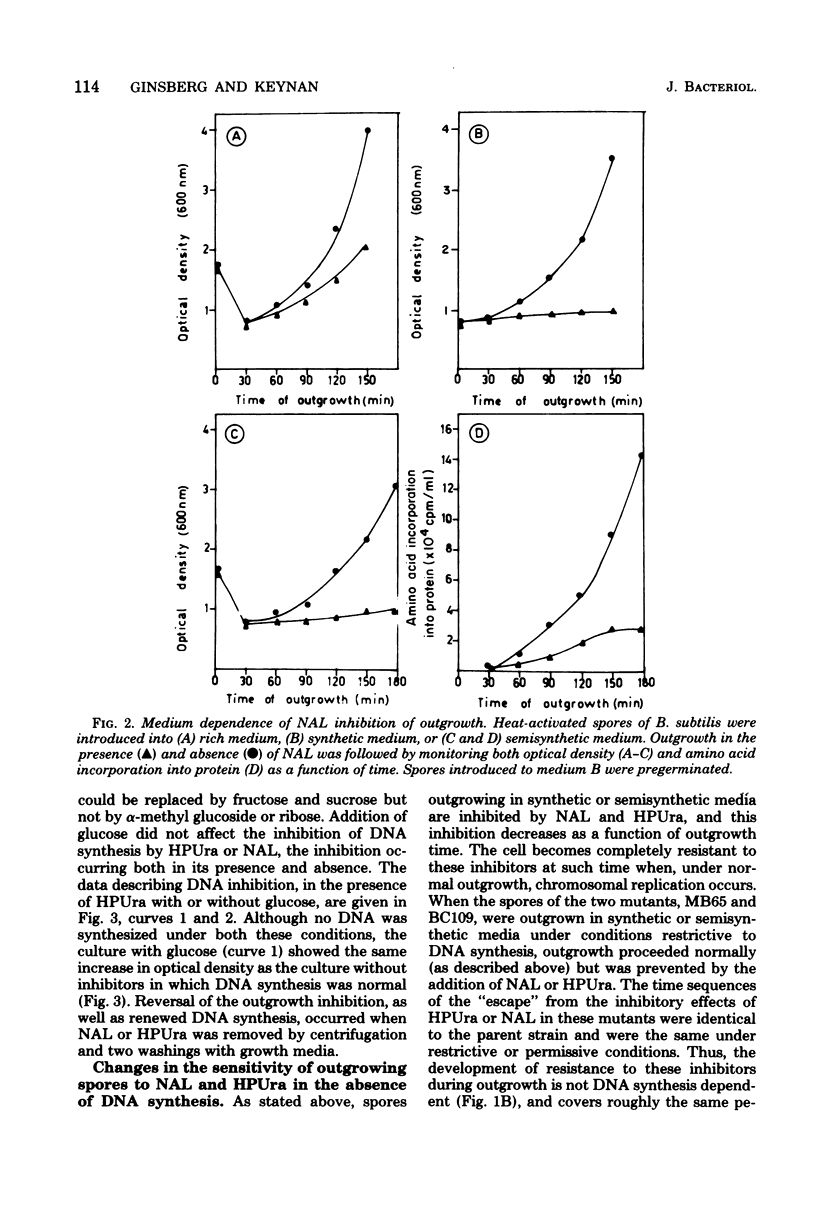
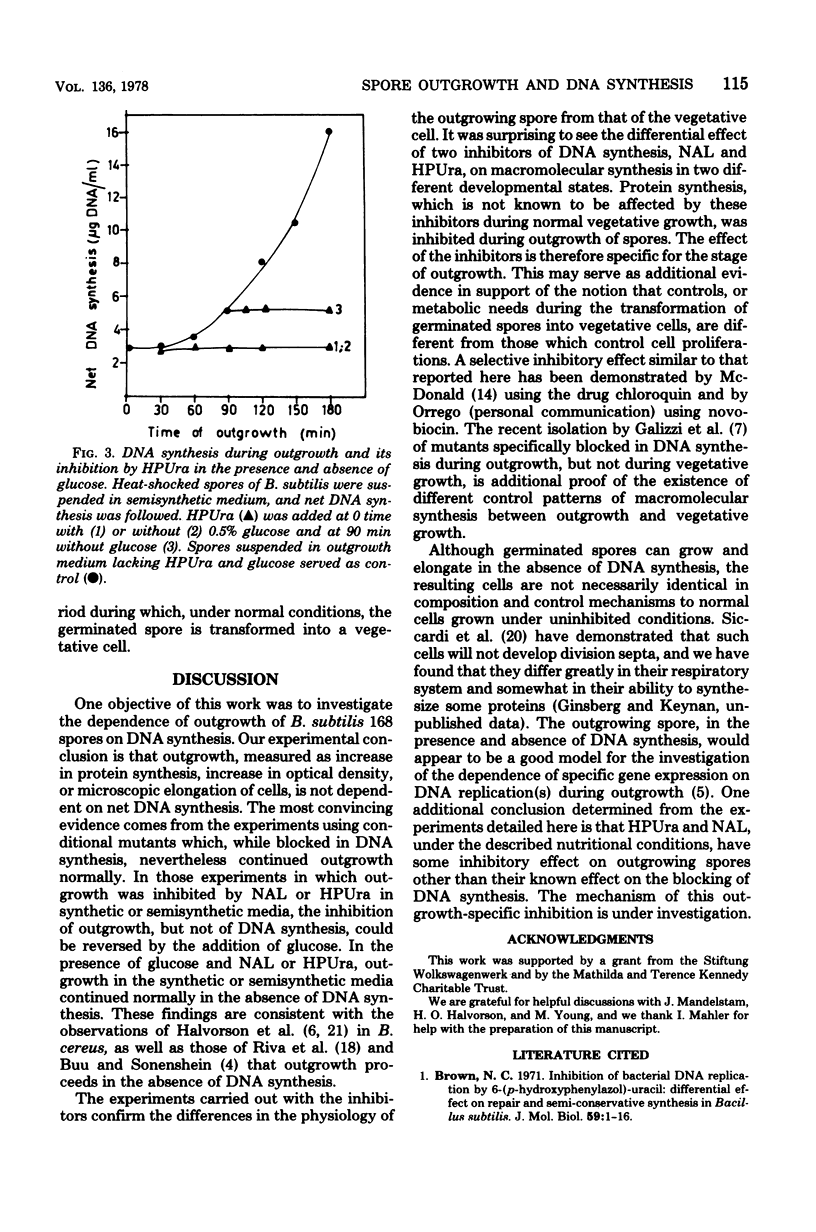
The outgrowth of spores of Bacillus subtilis 168 proceeded normally in temperature-sensitive DNA mutants under restrictive conditions and in the absence of DNA synthesis. Two inhibitors of DNA synthesis, nalidoxic acid and 6-(p-hydroxyphenylazo)-uracil, inhibited spore outgrowth under some nutritional conditions; this inhibition of outgrowth however, though not that of DNA synthesis, could be reversed by glucose. The sensitivity of the outgrowing spores to nalidixic acid and 6-(p-hydroxyphenylazo)-uracil inhbition decreased as a function of outgrowth time. The cells became completely resistant to the inhibitors after 90 min. The development of this resistance occurred also in the absence of DNA synthesis. It was concluded that DNA synthesis is not needed for spore outgrowth, and that outgrowing cells and vegetative cells differ in their sensitivity to these inhibitors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Brown N. C., Wisseman C. L., 3rd, Matsushita T. Inhibition of bacterial DNA replication by 6-(p-hydroxy-phenylazo)-uracil. Nat New Biol. 1972 May 17;237(72):72–74. doi: 10.1038/newbio237072a0. [DOI] [PubMed] [Google Scholar]
- Buu A., Sonenshein A. L. Nucleic acid synthesis and ribonucleic acid polymerase specificity in germinating and outgrowing spores of Bacillus subtilis. J Bacteriol. 1975 Oct;124(1):190–200. doi: 10.1128/jb.124.1.190-200.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Cell cycle dependency of sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Mar;109(3):1027–1033. doi: 10.1128/jb.109.3.1027-1033.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynan A., Berns A. A., Dunn G., Young M., Mandelstam J. Resporulation of outgrowing Bacillus subtilis spores. J Bacteriol. 1976 Oct;128(1):8–14. doi: 10.1128/jb.128.1.8-14.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J., Higgs S. A. Induction of sporulation during synchronized chromosome replication in Bacillus subtilis. J Bacteriol. 1974 Oct;120(1):38–42. doi: 10.1128/jb.120.1.38-42.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W. C. Inhibition of spore outgrowth in Bacillus subtilis by chloroquin. Can J Microbiol. 1967 May;13(5):611–613. doi: 10.1139/m67-078. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Halvorson H. O. Nature of deoxyribonucleic acid synthesis and its relationship to protein synthesis during outgrowth of Bacillus cereus T. J Bacteriol. 1972 Feb;109(2):606–615. doi: 10.1128/jb.109.2.606-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., van Sluis C., Mastromei G., Attolini C., Mazza G., Polsinelli M., Falaschi A. A new mutant of Bacillus subtilis altered in the initiation of chromosome replication. Mol Gen Genet. 1975;137(3):185–202. doi: 10.1007/BF00333015. [DOI] [PubMed] [Google Scholar]
- Setlow P. Deoxyribonucleic acid synthesis and deoxynucleotide metabolism during bacterial spore germination. J Bacteriol. 1973 Jun;114(3):1099–1107. doi: 10.1128/jb.114.3.1099-1107.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siccardi A. G., Galizzi A., Mazza G., Clivio A., Albertini A. M. Synchronous germination and outgrowth of fractionated Bacillus subtilis spores: tool for the analysis of differentiation and division of bacterial cells. J Bacteriol. 1975 Jan;121(1):13–19. doi: 10.1128/jb.121.1.13-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. II. Relationship between ordered enzyme synthesis and deoxyribonucleic acid replication. J Bacteriol. 1968 Feb;95(2):479–489. doi: 10.1128/jb.95.2.479-489.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESE C. R., FORRO J. R. Correlations between ribonucleic acid and deoxyribonucleic acid metabolism during spore germination. J Bacteriol. 1960 Dec;80:811–817. doi: 10.1128/jb.80.6.811-817.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winshell E. B., Rosenkranz H. S. Nalidixic Acid and the Metabolism of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1168–1175. doi: 10.1128/jb.104.3.1168-1175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


