Abstract
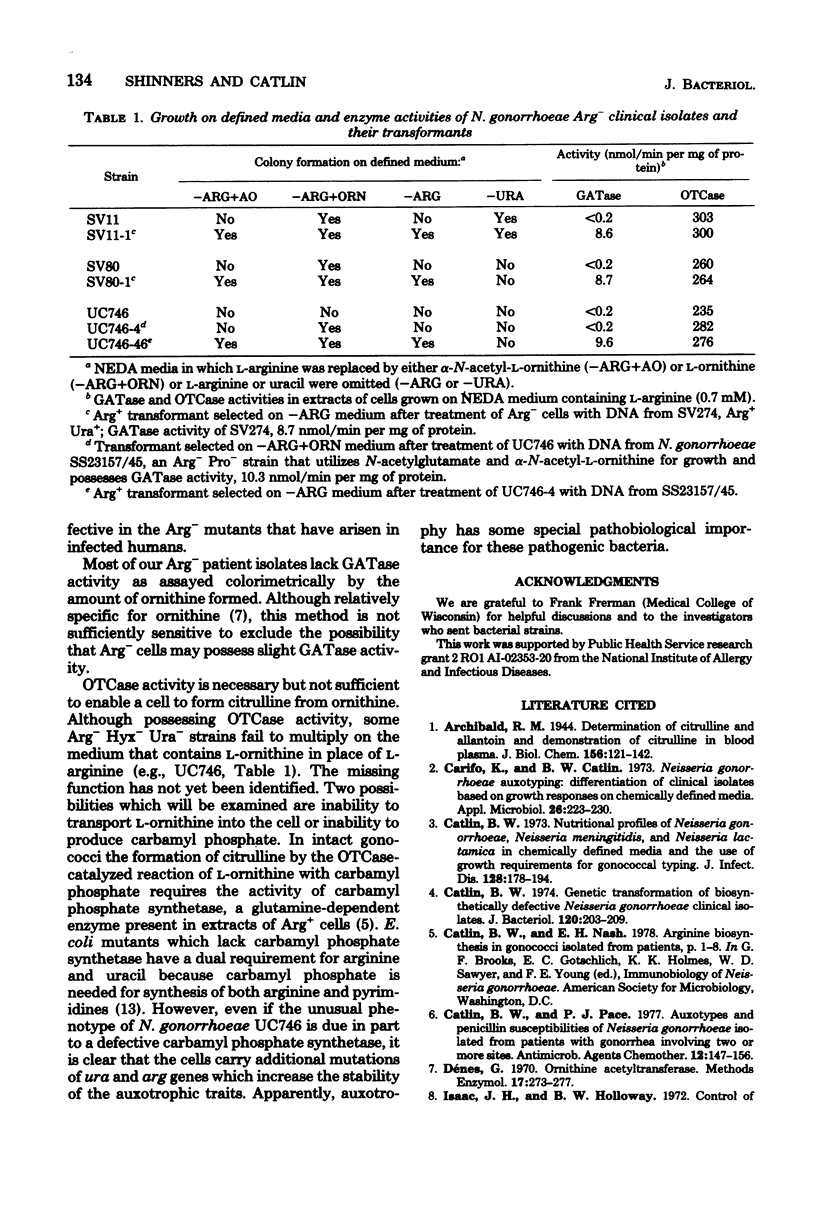
Many of the Neisseria gonorrhoeae strains isolated from patients require arginine for growth in a defined medium. As a basis for genetic studies of these Arg- strains, we examined two biosynthetic enzymes of Arg+ (nonrequiring) gonococci. Cell-free extracts contained (i) glutamate acetyltransferase, which catalyzes the formation of L-ornithine from alpha-N-acetyl-L-ornithine, and (ii) ornithine transcaramylase, which catalyzes the reaction between L-ornithine and carbamyl phosphate, yielding L-citrulline. Arg- strains were unable to utilze alpha-N-acetyl-L-ornithine for growth lacked significant activity of glutamate acetyltransferase, and activity was gained by Arg+ clones derived by DNA-mediated transformation. Some of the Arg- patient isolates were unable to use either alpha-N-acetyl-L-ornithine or L-ornithine in place of arginine, and two separate steps of genetic transformation were required to yield Arg+ cells. Extracts of these doubly auxotrophic cells lacked glutamate acetyltransferase activity, but, unexpectedly, they displayed normal ornithine transcarbamylase activity. This finding illustrates the importance of identifying the products specified by arg loci during genetic studies of arginine auxotrophy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carifo K., Catlin B. W. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973 Sep;26(3):223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Genetic transformation of biosynthetically defective Neisseria gonorrhoeae clinical isolates. J Bacteriol. 1974 Oct;120(1):203–209. doi: 10.1128/jb.120.1.203-209.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Catlin B. W., Pace P. J. Auxotypes and penicillin susceptibilities of Neisseria gonorrhoeae isolated from patients with gonorrhea involving two or more sites. Antimicrob Agents Chemother. 1977 Aug;12(2):147–156. doi: 10.1128/aac.12.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J. H., Holloway B. W. Control of arginine biosynthesis in Pseudomonas aeruginosa. J Gen Microbiol. 1972 Dec;73(3):427–438. doi: 10.1099/00221287-73-3-427. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Mapping the gene determining ornithine transcarbamylase and its operator in Escherichia coli B. J Bacteriol. 1971 Nov;108(2):645–651. doi: 10.1128/jb.108.2.645-651.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayer L. W., Schoolnik G. K., Falkow S. Genetic studies on Neisseria gonorrhoeae from disseminated gonococcal infections. Infect Immun. 1977 Oct;18(1):165–172. doi: 10.1128/iai.18.1.165-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergeay M., Gigot D., Beckmann J., Glansdorff N., Piérard A. Physiology and genetics of carbamoylphosphate synthesis in Escherichia coli K12. Mol Gen Genet. 1974;133(4):299–316. doi: 10.1007/BF00332706. [DOI] [PubMed] [Google Scholar]
- Staub M., Dénes G. Mechanism of arginine biosynthesis in Chlamydomonas reinhardti. I. Purification and properties of ornithine acetyltransferase. Biochim Biophys Acta. 1966 Oct 17;128(1):82–91. doi: 10.1016/0926-6593(66)90144-5. [DOI] [PubMed] [Google Scholar]
- Udaka S. Pathway-specific pattern of control of arginine biosynthesis in bacteria. J Bacteriol. 1966 Feb;91(2):617–621. doi: 10.1128/jb.91.2.617-621.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vogel H. J., Vogel R. H. Enzymes of arginine biosynthesis and their repressive control. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):65–90. doi: 10.1002/9780470122853.ch3. [DOI] [PubMed] [Google Scholar]


