Abstract
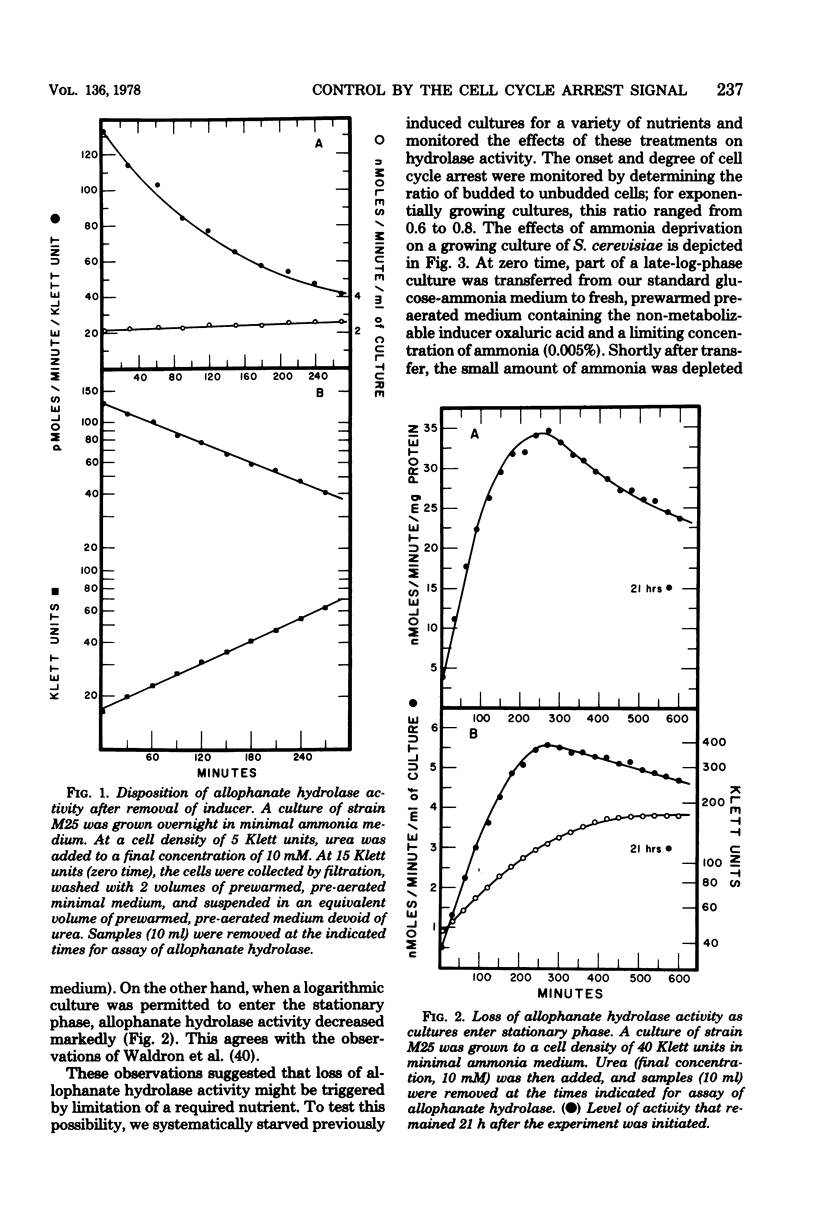
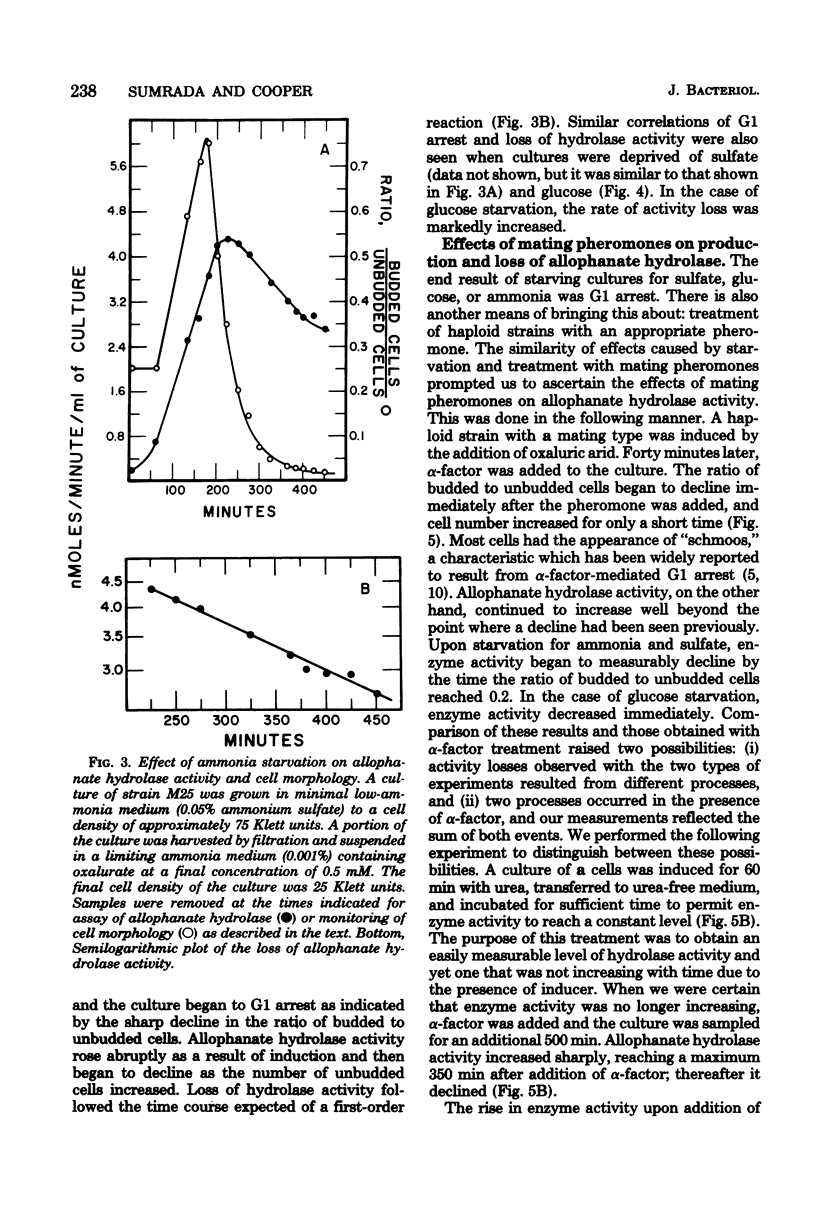
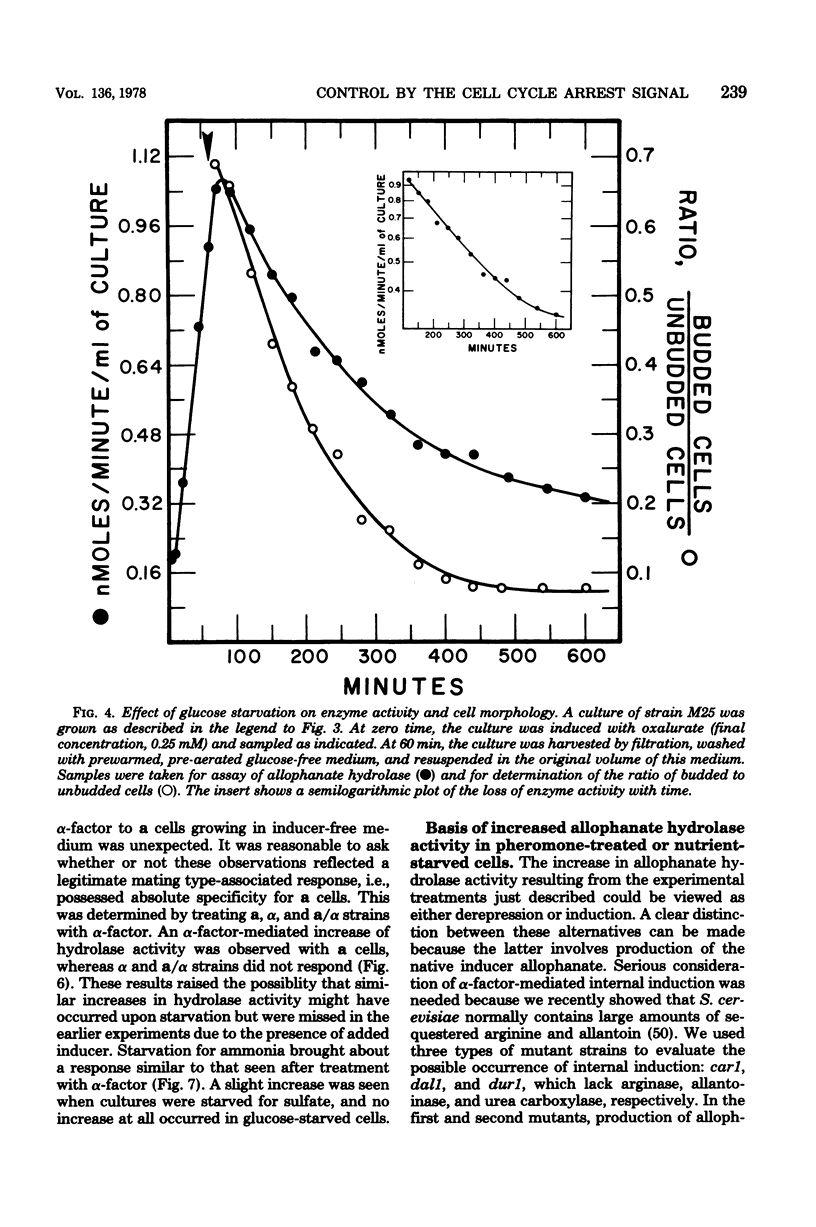
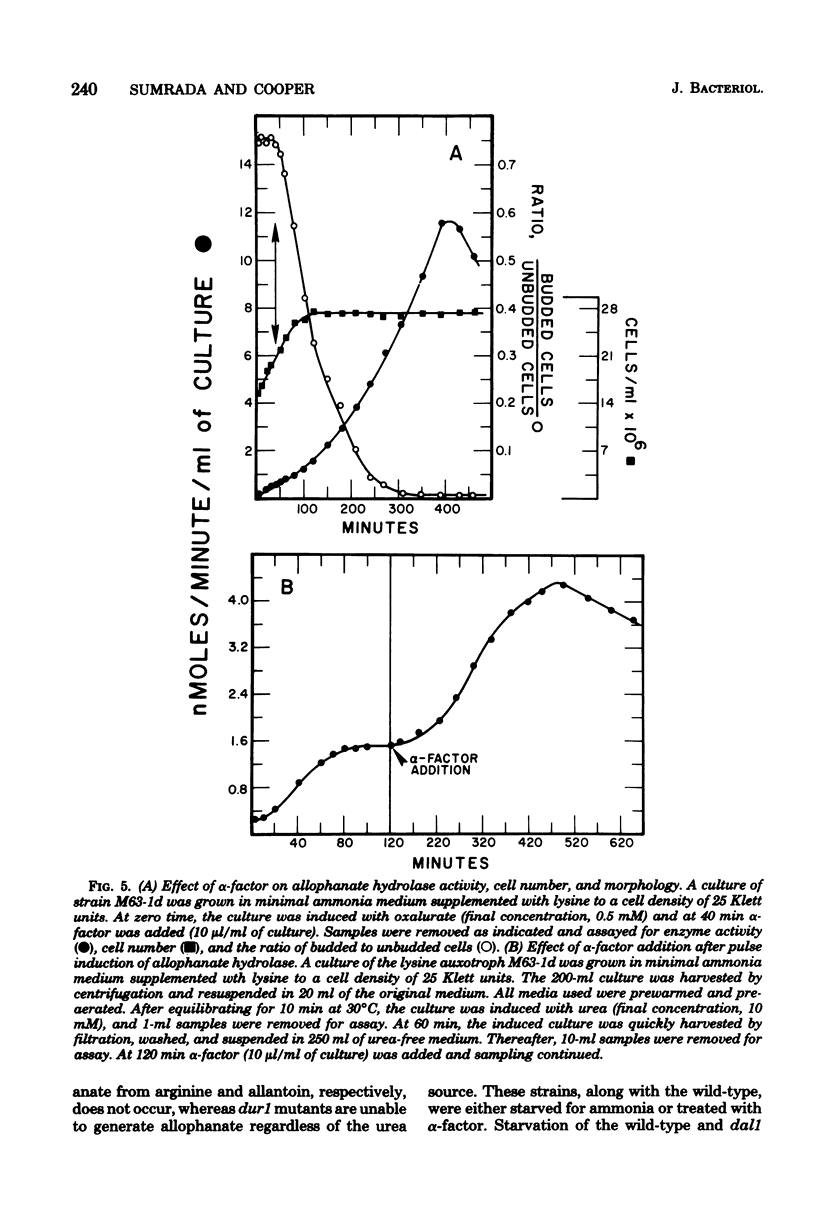
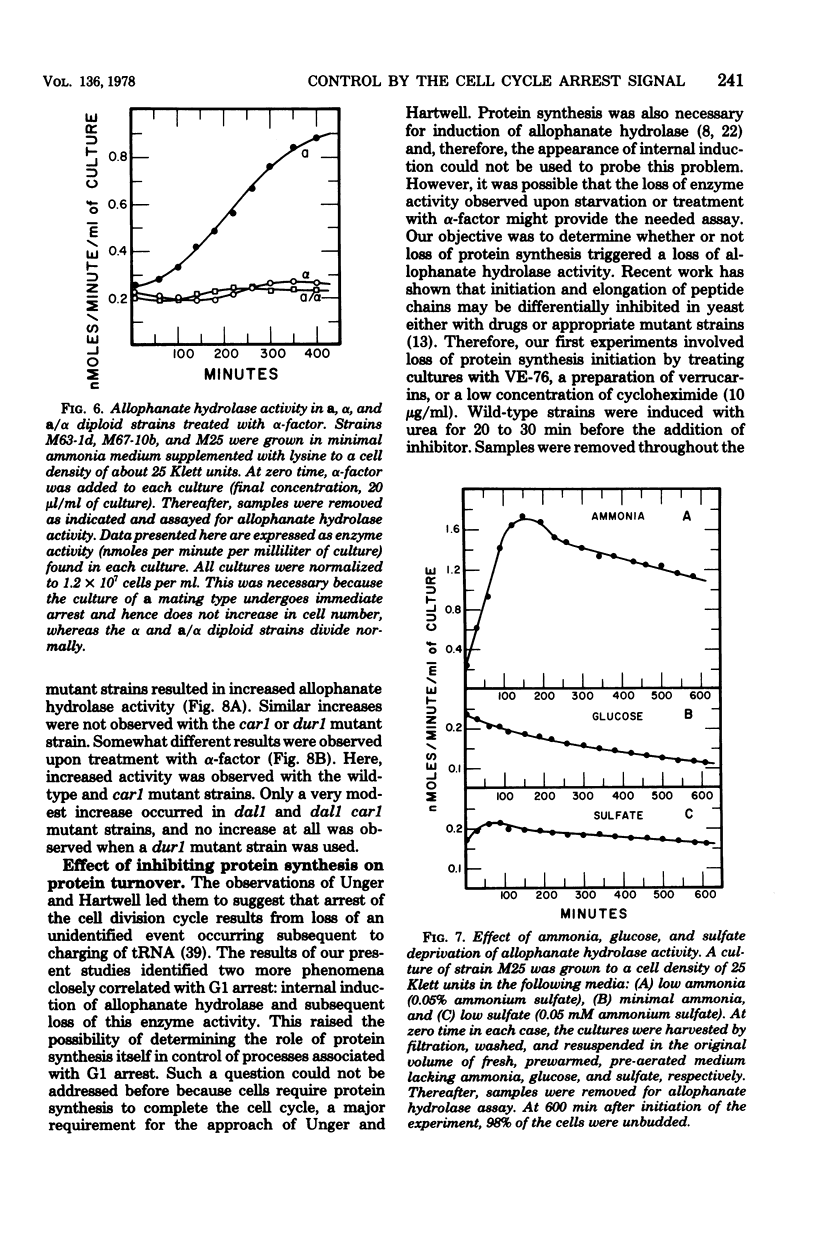
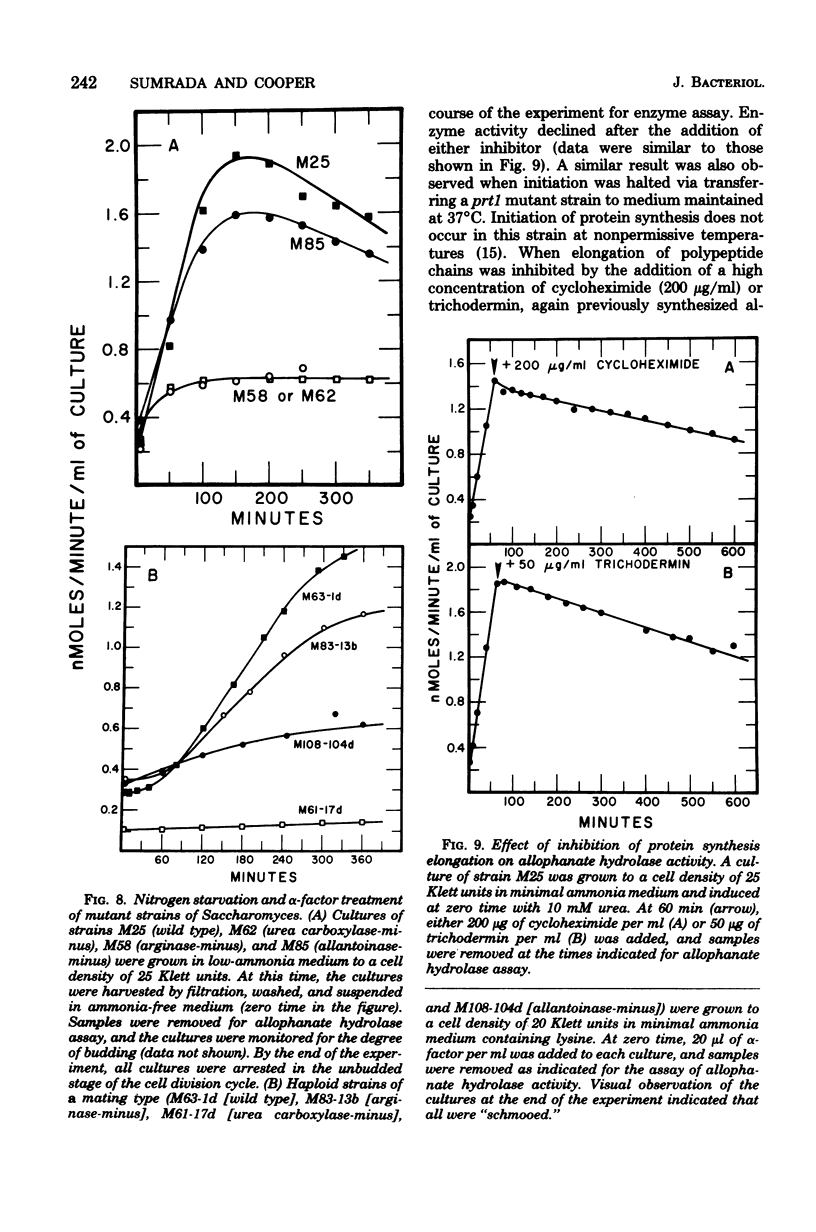
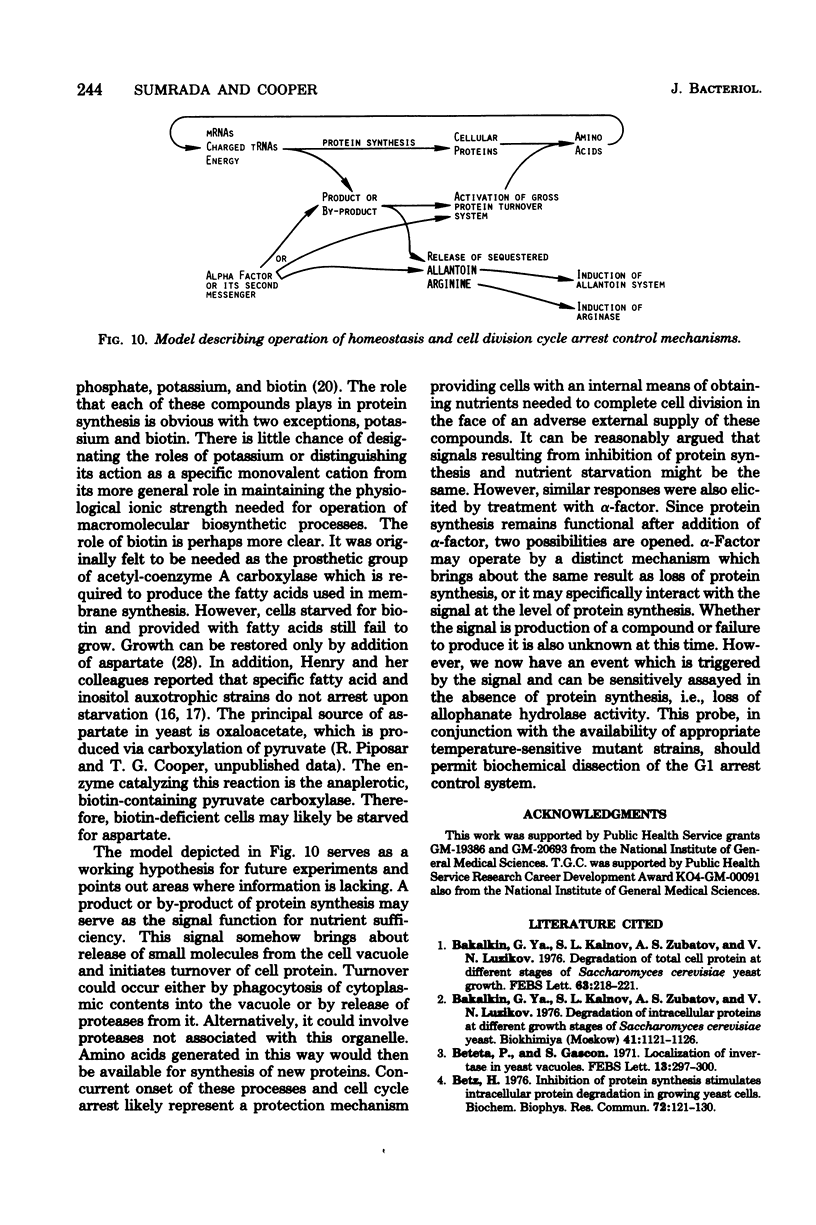
Saccharomyces cerevisiae responds to deperivation of nutrients by arresting cell division at the unbudded G1 stage. Cells situated outside of G1 at the time of deperivation complete the cell cycle before arresting. This prompted an investigation of the source of nutrients used by these cells to complete division and the mechanisms controlling their availability. We found a close correlation between accumulation of unbudded cells and loss of previously formed allophanate hydrolase activity after nutrient starvation. These losses were not specific to the allantoin, system since they have been observed for a number of other enzymes and also when cellular protein levels were monitored with [3H]leucine. Loss of hydrolase activity was also observed when protein synthesis was inhibited either by addition of inhibitors or loss of the prtl gene product. We found that onset of nutrient starvation brought about release of large quantities of arginine and allantoin normally sequestered in the cell vacuole. Treatment of a cells with alpha-factor resulted in both the release of allantoin and arginine from the cell vacuole and the onset of intracellular protein degradation. These effects were not observed when either alpha cells or a/alpha diploid strains were treated with alpha-factor. These data suggest that release of vacuolar constitutents and protein turnover may be regulated by the G1 arrest signal.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakalkin G. Y., Kalnov S. L., Zubatov A. S., Luzikov V. N. Degradation of total cell protein at different stages of Saccharomyces cerevisiae yeast growth. FEBS Lett. 1976 Mar 15;63(1):218–221. doi: 10.1016/0014-5793(76)80231-1. [DOI] [PubMed] [Google Scholar]
- Beteta P., Gascon S. Localization of invertase in yeast vacuoles. FEBS Lett. 1971 Mar 22;13(5):297–300. doi: 10.1016/0014-5793(71)80245-4. [DOI] [PubMed] [Google Scholar]
- Betz H. Inhibition of protein synthesis stimulates intracellular protein degradation in growing yeast cells. Biochem Biophys Res Commun. 1976 Sep 7;72(1):121–130. doi: 10.1016/0006-291x(76)90969-4. [DOI] [PubMed] [Google Scholar]
- Betz H., Weiser U. Protein degradation during yeast sporulation. Enzyme and cytochrome patterns. Eur J Biochem. 1976 Nov 15;70(2):385–395. doi: 10.1111/j.1432-1033.1976.tb11028.x. [DOI] [PubMed] [Google Scholar]
- Betz H., Weisner U. Protein degradation and proteinases during yeast sporulation. Eur J Biochem. 1976 Feb 2;62(1):65–76. doi: 10.1111/j.1432-1033.1976.tb10098.x. [DOI] [PubMed] [Google Scholar]
- Bossinger J., Cooper T. G. Sequence of molecular events involved in induction of allophanate hydrolase. J Bacteriol. 1976 Apr;126(1):198–204. doi: 10.1128/jb.126.1.198-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger J., Lawther R. P., Cooper T. G. Nitrogen repression of the allantoin degradative enzymes in Saccharomyces cerevisiae. J Bacteriol. 1974 Jun;118(3):821–829. doi: 10.1128/jb.118.3.821-829.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücking-Throm E., Duntze W., Hartwell L. H., Manney T. R. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973 Jan;76(1):99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. Yeast chitin synthetase. Separation of the zymogen from its activating factor and recovery of the latter in the vacuole fraction. J Biol Chem. 1973 Feb 25;248(4):1451–1458. [PubMed] [Google Scholar]
- Chan R. K. Recovery of Saccharomyces cerevisiae mating-type a cells from G1 arrest by alpha factor. J Bacteriol. 1977 May;130(2):766–774. doi: 10.1128/jb.130.2.766-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Bossinger J. Selective inhibition of protein synthesis initiation in Saccharomyces cerevisiae by low concentrations of cycloheximide. J Biol Chem. 1976 Nov 25;251(22):7278–7280. [PubMed] [Google Scholar]
- Duntze W., Stötzler D., Bücking-Throm E., Kalbitzer S. Purification and partial characterization of -factor, a mating-type specific inhibitor of cell reproduction from Saccharomyces cerevisiae. Eur J Biochem. 1973 Jun;35(2):357–365. doi: 10.1111/j.1432-1033.1973.tb02847.x. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A. Death resulting from fatty acid starvation in yeast. J Bacteriol. 1973 Dec;116(3):1293–1303. doi: 10.1128/jb.116.3.1293-1303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indge K. J. Polyphosphates of the yeast cell vacuole. J Gen Microbiol. 1968 May;51(3):447–455. doi: 10.1099/00221287-51-3-447. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A., McFarlane S. Growth and cell division during nitrogen starvation of the yeast Saccharomyces cerevisiae. J Bacteriol. 1977 Nov;132(2):723–730. doi: 10.1128/jb.132.2.723-730.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Kinetics of induced and repressed enzyme synthesis in Saccharomyces cerevisiae. J Bacteriol. 1975 Mar;121(3):1064–1073. doi: 10.1128/jb.121.3.1064-1073.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Riemer E., Chojnacki B., Cooper T. G. Clustering of the genes for allantoin degradation in Saccharomyces cerevisiae. J Bacteriol. 1974 Aug;119(2):461–468. doi: 10.1128/jb.119.2.461-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Mazón M. J. Effect of glucose starvation on the nicotinamide adenine dinucleotide phosphate-dependent glutamate dehydrogenase of yeast. J Bacteriol. 1978 Feb;133(2):780–785. doi: 10.1128/jb.133.2.780-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Cooper T. G. Evidence that specific and "general" control of ornithine carbamoyltransferase production occurs at the level of transcription in Saccharomyces cerevisiae. J Bacteriol. 1977 Jun;130(3):1253–1261. doi: 10.1128/jb.130.3.1253-1261.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUSH A. H. Crystallization of purines in the vacuole of Candida utilis. Nature. 1961 Apr 29;190:449–449. doi: 10.1038/190449a0. [DOI] [PubMed] [Google Scholar]
- Reid B. J., Hartwell L. H. Regulation of mating in the cell cycle of Saccharomyces cerevisiae. J Cell Biol. 1977 Nov;75(2 Pt 1):355–365. doi: 10.1083/jcb.75.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., DAINKO J. L., SCHLENK F. ULTRAVIOLET MICROSCOPY OF THE VACUOLE OF SACCHAROMYCES CEREVISIAE DURING SPORULATION. J Bacteriol. 1964 Aug;88:449–456. doi: 10.1128/jb.88.2.449-456.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., SCHLENK F. S-adenosylmethionine in the vacuole of Candida utilis. J Bacteriol. 1960 Jun;79:841–848. doi: 10.1128/jb.79.6.841-848.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk F., Dainko J. L., Svihla G. The accumulation and intracellular distribution of biological sulfoninum compounds in yeast. Arch Biochem Biophys. 1970 Sep;140(1):228–236. doi: 10.1016/0003-9861(70)90027-5. [DOI] [PubMed] [Google Scholar]
- Sims A. P., Toone J., Box V. The regulation of glutamine metabolism in Candida utilis: mechanisms of control of glutamine synthetase. J Gen Microbiol. 1974 Sep;84(1):149–162. doi: 10.1099/00221287-84-1-149. [DOI] [PubMed] [Google Scholar]
- Stötzler D., Betz R., Duntze W. Stimulation of yeast mating hormone activity by synthetic oligopeptides. J Bacteriol. 1977 Oct;132(1):28–35. doi: 10.1128/jb.132.1.28-35.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throm E., Duntze W. Mating-Type-Dependent Inhibition of Deoxyribonucleic Acid Synthesis in Saccharomyces cerevisiae. J Bacteriol. 1970 Dec;104(3):1388–1390. doi: 10.1128/jb.104.3.1388-1390.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger M. W., Hartwell L. H. Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1664–1668. doi: 10.1073/pnas.73.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron C., Jund R., Lacroute F. Evidence for a high proportion of inactive ribosomes in slow-growing yeast cells. Biochem J. 1977 Dec 15;168(3):409–415. doi: 10.1042/bj1680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G., Magasanik B. The induction of urea carboxylase and allophanate hydrolase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6203–6209. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Urea carboxylase and allophanate hydrolase. Two components of adenosine triphosphate:urea amido-lyase in Saccharomyces cerevisiae. J Biol Chem. 1972 Mar 10;247(5):1349–1353. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Urea carboxylase and allophanate hydrolase: two components of a multienzyme complex in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1972 Oct 6;49(1):45–51. doi: 10.1016/0006-291x(72)90007-1. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Magasanik B. The induction of arginase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6197–6202. [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]
- Zacharski C. A., Cooper T. G. Metabolite compartmentation in Saccharomyces cerevisiae. J Bacteriol. 1978 Aug;135(2):490–497. doi: 10.1128/jb.135.2.490-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



