Abstract
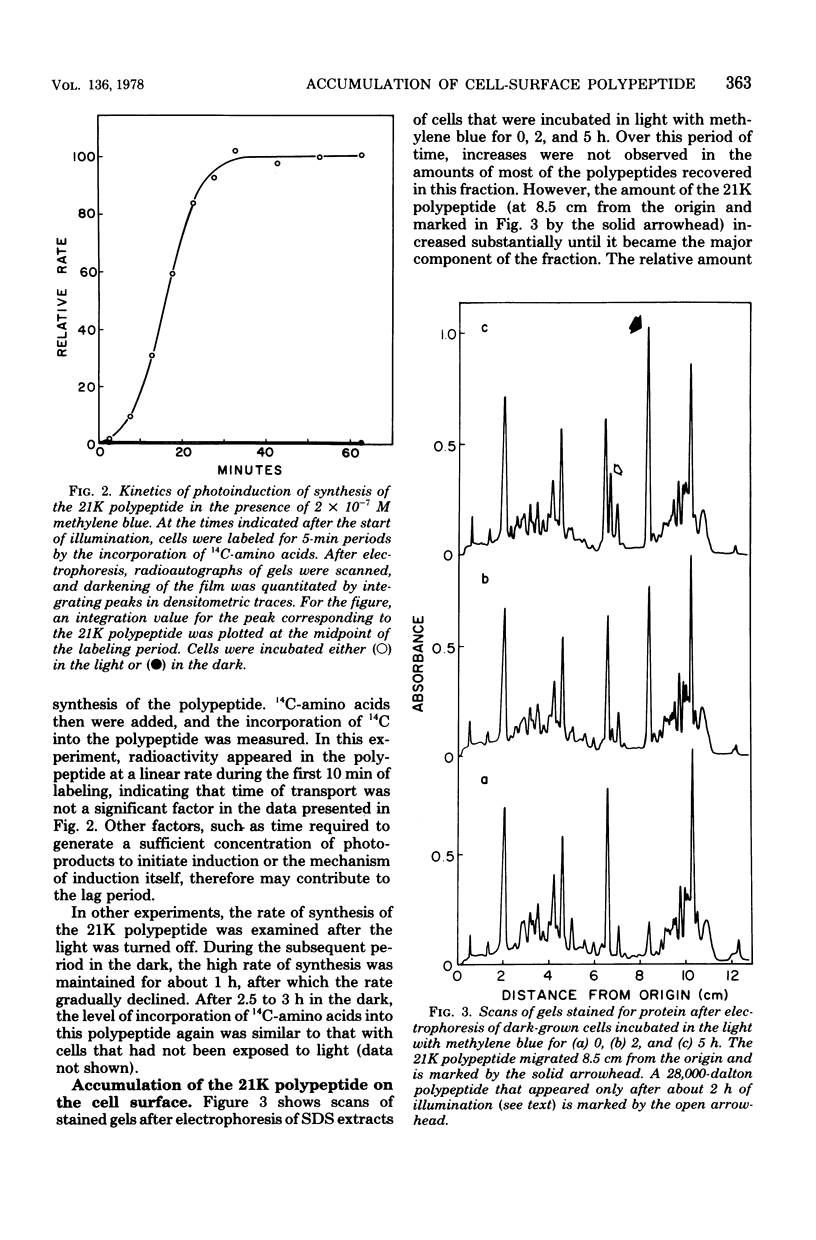
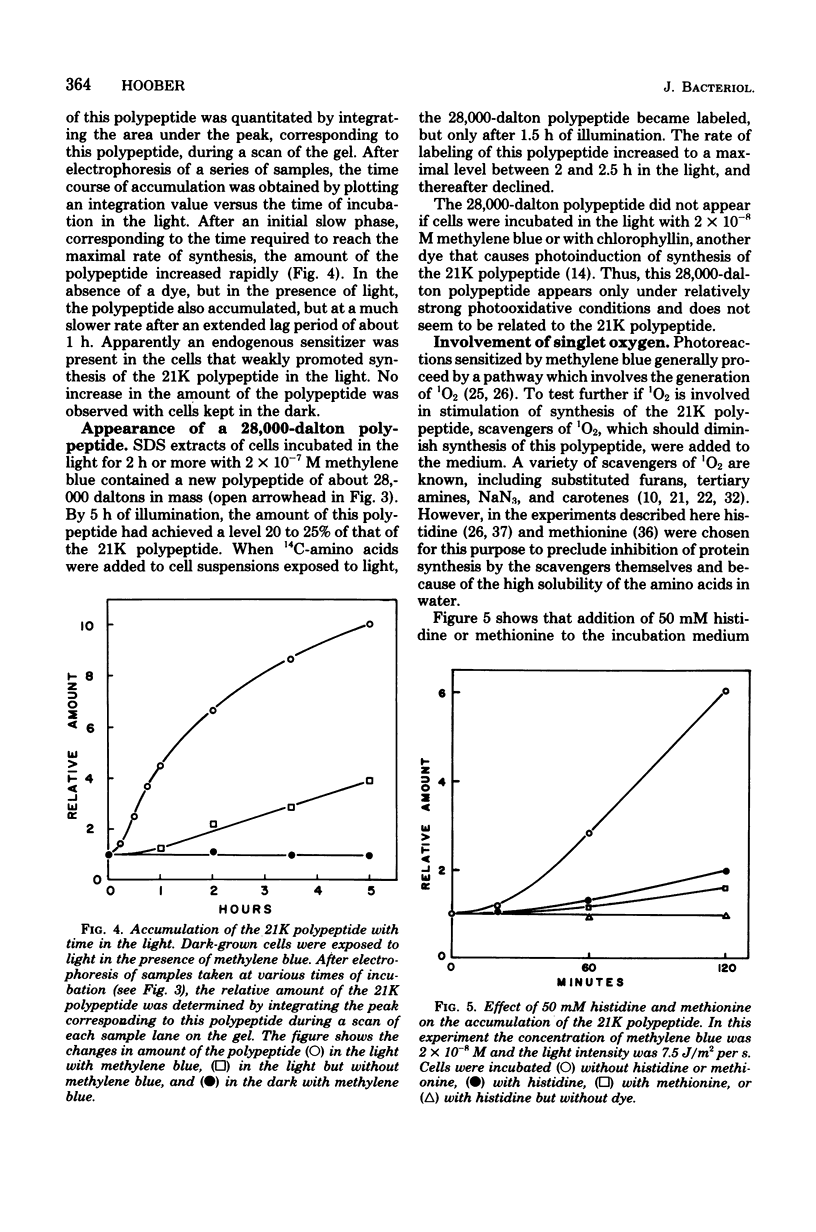
Cells of a species of Arthrobacter were incubated in the light with methylene blue, a dye that sensitizes photooxidative reactions by the production of singlet oxygen. An early and major response by the cells to these conditions was stimulation of synthesis of a single cell-surface polypeptide, 21,000 daltons in mass. The rate of synthesis of this polypeptide reached a maximal level about 30 min after the start of illumination. As a consequence, the amount of this polypeptide increased at least 10-fold during a period of 5 h. The presence of histidine or methionine, scavengers of singlet oxygen, markedly diminished synthesis and accumulation of this polypeptide. Concomitant with the accumulation of this polypeptide on the cell surface was the appearance of an extensive array of pili.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Bonneau R., Pottier R., Bagno O., Joussot-Dubien J. pH dependence of singlet oxygen production in aqueous solutions using thiazine dyes as photosensitizers. Photochem Photobiol. 1975 Mar;21(3):159–163. doi: 10.1111/j.1751-1097.1975.tb06646.x. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Fiedler F., Schleifer K., Kandler O. Amino acid sequence of the threonine-containing mureins of coryneform bacteria. J Bacteriol. 1973 Jan;113(1):8–17. doi: 10.1128/jb.113.1.8-17.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote C. S. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968 Nov 29;162(3857):963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W. Composition and molecular weight of pili purified from Pseudomonas aeruginosa K. J Bacteriol. 1977 Jul;131(1):259–269. doi: 10.1128/jb.131.1.259-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda E., Yanagawa R. Agglutination of trypsinized sheep erythrocytes by the pili of Corynebacterium renale. Infect Immun. 1974 Dec;10(6):1426–1432. doi: 10.1128/iai.10.6.1426-1432.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K. Photodynamic induction of a bacterial cell surface polypeptide. J Bacteriol. 1977 Aug;131(2):650–656. doi: 10.1128/jb.131.2.650-656.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACIN H., MISHKIN A. R. SEPARATION OF CARBOHYDRATES ON BORATE-IMPREGNATED SILICA GEL G PLATES. J Chromatogr. 1965 Apr;18:170–173. doi: 10.1016/s0021-9673(01)80341-1. [DOI] [PubMed] [Google Scholar]
- Jacob H. E., Hamann M. Photodynamic alterations of the cell envelope of Proteus mirabilis and their repair. Photochem Photobiol. 1975 Dec;22(6):237–241. doi: 10.1111/j.1751-1097.1975.tb06742.x. [DOI] [PubMed] [Google Scholar]
- Keddie N. C., Taylor A. W., Sykes P. A. The termination of the common bile duct. Br J Surg. 1974 Aug;61(8):623–625. doi: 10.1002/bjs.1800610808. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa N., Yanagawa R. Chemical properties of the pili of Corynebacterium renale. Infect Immun. 1972 Jan;5(1):27–30. doi: 10.1128/iai.5.1.27-30.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson R., Kearns D. R. A remarkable deuterium effect on the rate of photosensitized oxidation of alcohol dehydrogenase and trypsin. Photochem Photobiol. 1973 Jan;17(1):65–68. doi: 10.1111/j.1751-1097.1973.tb06333.x. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Merkel P. B., Kearns D. R. Unambiguous evidence for the participation of singlet oxygen ( 1 ) in photodynamic oxidation of amino acids. Photochem Photobiol. 1972 Aug;16(2):117–124. doi: 10.1111/j.1751-1097.1972.tb07343.x. [DOI] [PubMed] [Google Scholar]
- Robertson J. N., Vincent P., Ward M. E. The preparation and properties of gonococcal pili. J Gen Microbiol. 1977 Sep;102(1):169–177. doi: 10.1099/00221287-102-1-169. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Vadasz J. A. Singlet oxygen quenchers and the photodynamic inactivation of E. coli ribosomes by methylene blue. Biochem Biophys Res Commun. 1976 May 23;76(2):391–397. doi: 10.1016/0006-291x(77)90737-9. [DOI] [PubMed] [Google Scholar]
- Stegeman W. J., Hoober J. K. Induction of synthesis of bacterial protein by excretory product of the alga Chlamydomonas reinhardtii y-1. Nature. 1975 Sep 18;257(5523):244–246. doi: 10.1038/257244a0. [DOI] [PubMed] [Google Scholar]
- Wilson T., Hastings J. W. Chemical and biological aspects of singlet excited molecular oxygen. Photophysiology. 1970;5:49–95. [PubMed] [Google Scholar]
- Yaguchi M., Daoust V., Perry M. B. Ion-exchange chromatography of 2-amino-2,6-dideoxyhexoses and of the methyl ethers of 2-amino-2,6-dideoxy-D-glucopyranose (quinovosamine) and 2-amino-2,6-dideoxy-D-galactopyranose (fucosamine). Carbohydr Res. 1975 Jan;39(1):131–135. doi: 10.1016/s0008-6215(00)82645-2. [DOI] [PubMed] [Google Scholar]
- Yanagawa R., Honda E. Presence of pili in species of human and animal parasites and pathogens of the genuscorynebacterium. Infect Immun. 1976 Apr;13(4):1293–1295. doi: 10.1128/iai.13.4.1293-1295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




