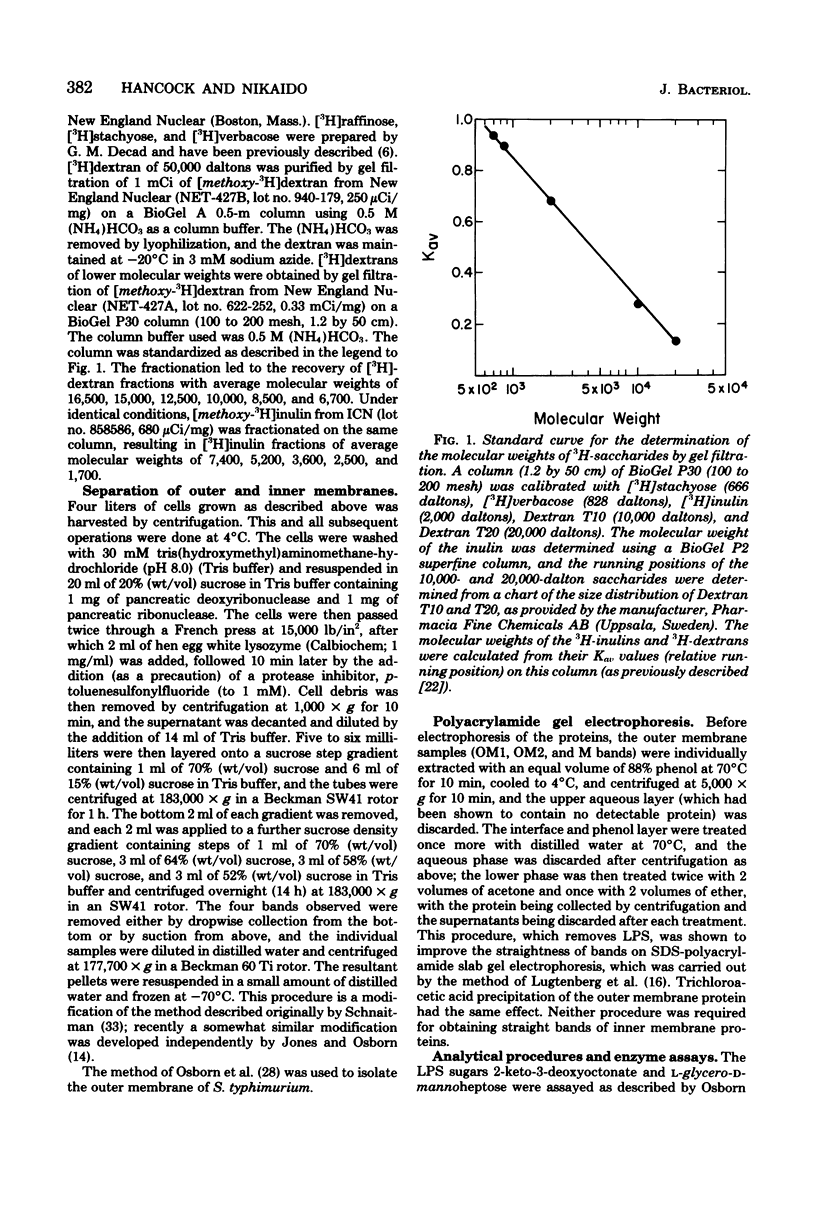
Abstract
A method for separating the outer and inner membranes of Pseudomonas aeruginosa PAO1 in the absence of added ethylenediaminetetraacetic acid was devised. The method yields two outer membrane fractions which show the same protein pattern on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, but differ substantially in their relative contents of phospholipids. One of these outer membrane fractions and the inner membrane fraction are less than 4% cross-contaminated, as judged by the content of typical inner and outer membrane markers. The outer membrane contains four major protein bands with apparent molecular weights of 37,000, 35,000, 21,000 and 17,000. Vesicles reconstituted from lipopolysaccharide and phospholipids were impermeable to all saccharides included in the vesicles during vesicle formation. When the vesicles contained outer membrane proteins, they fully retained only those saccharides of greater than 9,000 molecular weight, suggesting that the exclusion limit of the outer membrane of P. aeruginosa for saccharides is substantially larger than the figure (500 to 600 daltons) obtained for certain enteric bacteria. The advantages and potential disadvantages of having an outer membrane with a higher exclusion limit for hydrophilic substances are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth B. R., Curtis N. A. Separation of the cytoplasmic and outer membrane of Pseudomonas aeruginosa PAQ. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1168–1176. doi: 10.1016/0006-291x(77)91641-2. [DOI] [PubMed] [Google Scholar]
- Chester I. R., Gray G. W., Wilkinson S. G. Further studies of the chemical composition of the lipopolysaccharide of Pseudomonas aeruginosa. Biochem J. 1972 Jan;126(2):395–407. doi: 10.1042/bj1260395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Murray R. G. Ultrastructural study of polymyxin-resistant isolates of Pseudomonas aeruginosa. J Bacteriol. 1976 Jan;125(1):267–281. doi: 10.1128/jb.125.1.267-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Roth I. L., Eagon R. G. Freeze-etch study of Pseudomonas aeruginosa: localization within the cell wall of an ethylenediaminetetraacetate-extractable. J Bacteriol. 1973 Jan;113(1):417–432. doi: 10.1128/jb.113.1.417-432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock I. C., Meadow P. M. The extractable lipids of Pseudomonas aeruginosa. Biochim Biophys Acta. 1969 Oct 28;187(3):366–379. doi: 10.1016/0005-2760(69)90010-1. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Johnson G. G., Morris J. M., Berk R. S. The extracellular protease from Pseudomonas aeruginosa exhibiting elastase activity. Can J Microbiol. 1967 Jun;13(6):711–719. doi: 10.1139/m67-093. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- Kasahara M., Anraku Y. Succinate dehydrogenase of Escherichia coli membrane vesicles. Activation and properties of the enzyme. J Biochem. 1974 Nov;76(5):959–966. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Adachi O., Shinagawa E., Ameyama M. Isolation and characterization of outer and inner membranes from Pseudomonas aeruginosa and effect of EDTA on the membranes. J Biochem. 1978 Jan;83(1):171–181. doi: 10.1093/oxfordjournals.jbchem.a131888. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Becker J. M. Peptide utilization in Pseudomonas aeruginosa: evidence for membrane-associated peptidase. J Bacteriol. 1978 Jan;133(1):165–171. doi: 10.1128/jb.133.1.165-171.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella typhimurium: reconstitution of sucrose-permeable membrane vesicles. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1224–1230. doi: 10.1016/0006-291x(75)90823-2. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- Nordström K., Sykes R. B. Effects of sublethal concentrations of benzylpenicillin on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Dec;6(6):741–746. doi: 10.1128/aac.6.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Two-dimensional SDS-polyacrylamide gel electrophoresis of heat-modifiable outer-membrane proteins. Can J Microbiol. 1976 Jan;22(1):83–91. doi: 10.1139/m76-011. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Eagon R. G. A model system for studying protein-lipopolysaccharide synthesis, assembly, and insertion in the outer membrane of Pseudomonas aeruginosa. Can J Microbiol. 1975 Nov;21(11):1834–1841. doi: 10.1139/m75-266. [DOI] [PubMed] [Google Scholar]
- Tseng J. T., Bryan L. E. The effect of complement and other cell wall reagents on tetracycline and streptomycin resistance in Pseudomonas aeruginosa. Can J Microbiol. 1974 Aug;20(8):1101–1107. doi: 10.1139/m74-172. [DOI] [PubMed] [Google Scholar]