Abstract
Humans are genetically unable to produce the sialic acid N-glycolylneuraminic acid (Neu5Gc), because of a mutation that occurred after our last common ancestor with great apes. Although Neu5Gc is presumed absent from normal humans, small amounts have been claimed to exist in human tumors and fetal meconium. We have generated an antibody with high specificity and avidity for Neu5Gc. Fetal tissues, normal adult tissues, and breast carcinomas from humans showed reactivity to this antibody, primarily within secretory epithelia and blood vessels. The presence of small amounts of Neu5Gc was confirmed by MS. Absent any known alternate pathway for its synthesis, we reasoned that these small amounts of Neu5Gc might originate from exogenous sources. Indeed, human cells fed with Neu5Gc incorporated it into endogenous glycoproteins. When normal human volunteers ingested Neu5Gc, a portion was absorbed and eliminated in urine, and small quantities were incorporated into newly synthesized glycoproteins. Neu5Gc has never been reported in plants or microbes to our knowledge. We found that Neu5Gc is rare in poultry and fish, common in milk products, and enriched in red meats. Furthermore, normal humans have variable amounts of circulating IgA, IgM, and IgG antibodies against Neu5Gc, with the highest levels comparable to those of the previously known anti-α-galactose xenoreactive antibodies. This finding represents an instance wherein humans absorb and metabolically incorporate a nonhuman dietary component enriched in foods of mammalian origin, even while generating xenoreactive, and potentially autoreactive, antibodies against the same molecule. Potential implications for human diseases are briefly discussed.
Sialic acids (Sias) are nine-carbon sugars typically found as outermost units on the mammalian cellular glycocalyx and on secreted glycoproteins (1–4). The most common Sias are N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc). Cellular Neu5Gc is generated by hydroxylation of the sugar nucleotide donor CMP-Neu5Ac to CMP-Neu5Gc, catalyzed by CMP-Neu5Ac hydroxylase (CMAH) (5–7). Although Neu5Gc is a major Sia in most mammals (including our closest evolutionary relatives, the great apes) (8), it is thought to be absent in healthy humans (1–3). Indeed, humans generate immune responses against i.v.-administered molecules carrying Neu5Gc, e.g., the “serum sickness” reaction to equine antithymocyte globulin therapy (9, 10). These findings are explained by a human-specific inactivating mutation in the CMAH gene (11–13) that occurred ≈2.5 million to 3 million years ago (14).
Despite no known alternate pathway for Neu5Gc synthesis in humans, antibodies have been used to claim its presence in some human cancers (15–20) and human fetal meconium (15). However, the specificity of the polyclonal antibodies used was not well defined. One study using mAbs failed to detect Neu5Gc in human tumors and tissues (21). However, these mAbs were specific for Neu5Gc only in the context of underlying structural motifs. Another mAb thought to be specific for Neu5Gc cross-reacts with some sulfated glycolipids (22). Meanwhile, some reports claim chemical proof for Neu5Gc in human tumors (18, 19, 23).
Human biosynthetic pathways could theoretically allow exogenous Neu5Gc to be metabolically incorporated (13, 24). Indeed, human cells cultured in FCS express cell surface Neu5Gc in small amounts (8, 21). However, it is not known whether this expression represents passive adsorption of serum glycoconjugates or metabolic incorporation. Although earlier studies claimed the absence of Neu5Gc from normal human tissues, we noted a small HPLC peak at the elution time of Neu5Gc in extracts from human organs (8). Here we explore whether Neu5Gc is actually present in normal and/or pathological human tissues, if normal humans can take up Neu5Gc from dietary sources, and if we spontaneously express antibodies against it.
Materials and Methods
Western Blot Analysis. Serum proteins or total proteins extracted from Neu5Gc-fed or nonfed Caco-2 cells were either treated or sham-treated with 10 milliunits of Arthrobacter ureafaciens sialidase (EY Laboratories) in 100 mM sodium acetate, pH 5.5, at 37°C for 3 h. After SDS/PAGE, the separated proteins were transferred to nitrocellulose membrane, and binding of the anti-Neu5Gc antibody [1:10,000 in Tris-buffered saline with 0.1% Tween 20 (TBST)] was detected by using a secondary horseradish peroxidase-conjugated donkey anti-chicken IgY antibody (1:30,000 in TBST) and Supersignal West Pico (Pierce).
Immunohistochemistry Using the Chicken Anti-Neu5Gc Antibody. Human tissues were collected by an approved Institutional Review Board protocol, from autopsies or unused pathological material, frozen in OCT compound (Sakura Finetek, Torrance, CA), and archived at –70°C. Harold McClure (Yerkes Primate Center, Atlanta) generously provided frozen chimpanzee tissues. Frozen tissue sections were air-dried for 30 min and fixed in 10% buffered formalin for 30 min. Endogenous peroxidase activity was quenched, and nonspecific binding sites were blocked with 5% (Neu5Gc-free) human serum in PBS for 30 min. Sections were then incubated with the anti-Neu5Gc antibody in 5% human serum/PBS at a 1:200 dilution at room temperature for 2 h (see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org, for antibody production). After washing, horseradish peroxidase-conjugated donkey anti-chicken IgY antibody in 5% human serum/PBS at a 1:100 dilution was applied for 1 h. Control sections were incubated with normal IgY. Specific binding was detected by using the Nova Red substrate kit (Vector Laboratories), followed by hematoxylin counterstaining.
Detection of Neu5Gc by HPLC Analysis and MS. Human autopsy tissues (0.5 g) were subjected to sequential organic extractions and glycopeptides preparation (8). Sias in glycopeptides and lipid extracts were released by acid and purified by ion exchange chromatography (25), derivatized with 1,2-diamino-4,5-methylene dioxybenzene (DMB), and analyzed by HPLC (DMB-HPLC) and MS (14, 26).
Purification of Neu5Gc from Porcine Submaxillary Mucin. See Supporting Text.
Feeding of Human Epithelial Cells with Neu5Gc. Caco-2 cells (American Type Culture Collection, human epithelial cells isolated from a primary colon carcinoma) were propagated in α-MEM (GIBCO/Invitrogen) supplemented with 20% heat-inactivated FCS, at 37°C, 5% CO2. Before feeding, the cells were split and cultured for 4 days in α-MEM supplemented with 20% heat-inactivated premium human serum type AB (Irvine Scientific). Cells were then cultured in various concentrations of free Neu5Gc for 3 days at 37°C. Cells were then washed with cold PBS, scraped into 20 mM sodium phosphate, pH 7.5, and sonicated. The protein extract was quantified and analyzed by Western blot by using the anti-Neu5Gc antibody.
Human Neu5Gc Ingestion Study. Human subjects signed informed consent documents approved by the University of California at San Diego Institutional Review Board. They avoided animal foods for 2 days and all medications for 1 day, used a lanolin-free (Neu5Gc-free) shampoo for 2 days, and ingested only fruit juice on the morning of study. Subjects then drank 150 mg of porcine submaxillary mucin Sias (95% Neu5Gc) dissolved in 100 ml of water. For the next 6 h they drank a 1:1 mixture of fruit juice and soymilk (Sia-free) at ≈2ml/kg per h. Serum, urine, and saliva samples were obtained at multiple time points. Human saliva was collected by chewing parafilm, after washing the mouth out well with water. Urine volume and pH were recorded before centrifugation at 1,000 × g for 10 min and collection of the supernatant. All samples were stored frozen at –20°C. Head hairs (≈20) and/or facial hair trimmings (from men) were collected before and several days after Neu5Gc ingestion.
Purification and Analysis of Sias and N-Acylmannosamines from Human Samples. Human salivary mucin was precipitated by adjusting to pH 3 with 100 mM acetic acid, stirring at 4°C overnight, collected at 14,000 × g for 10 min, and washed twice with 1 ml of ice-cold PBS. Human hair clippings were washed in 100 mM ice-cold acetic acid for 2 h and crushed in a clean ceramic mortar. Bound Sias from saliva, hair, or serum were released with mild acid as above, filtered through Microcon 10 filters (Millipore), dried down, reconstituted in water, and analyzed for Sia content by DMB-HPLC.
Five-milliliter urine aliquots were diluted 5-fold and loaded onto 10-ml columns of AG50W-X2 resin (H+ form, Bio-Rad) equilibrated in water. The run-through and washes (2 × 10 ml of water) were pooled and loaded onto 10-ml columns of AG1 × 8 resin (Formate form, Bio-Rad) equilibrated in water. The combined run-through and washes (2 × 10 ml of water) were lyophilized. After washing with 5 vol of 10 mM formic acid, free Sias were eluted with 5 vol of 1 M formic acid, diluted 2-fold with water, and lyophilized.
The neutral fraction from urine was dissolved in water, and reduced peracetylated volatile derivatives of free sugars were prepared (27). Samples were dissolved in 10 μl of acetone, and gas-liquid chromatography for N-acylmannosamines was performed on 1-μl aliquots (27). Eluting ions were detected and fragmented by using a HP5971 Mass Selective Detector (Hewlett–Packard).
Detection of Anti-Neu5Gc and Anti-α-Galactose (α-Gal)-Specific Antibodies in Human Sera. Microtiter plate (Costar) wells were coated with Neu5Ac-polyacrylamide (PAA), Neu5Gc-PAA, or Galα1–3Galbeta1–4GlcNAc-PAA (Glycotech, Rockville, MD) at a concentration of 500 ng per well, in 50 mM sodium carbonate-bicarbonate buffer, pH 9.5, at 4°C overnight. After washing with TBS, pH 7.5, and blocking with TBST for2hat room temperature, triplicate wells were incubated with 1:50 dilutions of serum in TBST at room temperature for 4 h. Wells were washed five times with TBS and incubated with horseradish peroxidase-conjugated mouse antihuman IgA (Calbiochem), anti-human IgG (Jackson ImmunoResearch Laboratories), or anti-human IgM (Kirkegaard & Perry Laboratories) each diluted in TBST at 1:20,000, at room temperature for 1.5 h. The IgG anti-Neu5Gc and anti-α-Gal antibodies were quantitated by using a standard curve of normal human IgG coated to the wells under the same general conditions.
Purification and Analysis of Sias from Food Samples. One-gram portions of samples were homogenized in 2 M acetic acid, and total Sias were released at 80°C for 3 h. After clarification at 50,000 g, the supernatant was filtered through a Microcon 10 unit, dried down, reconstituted in water, de-O-acetylated with 0.1 M NaOH for 30 min at 37°C, and neutralized with HCl. Aliquots were derivatized with DMB, and Neu5Gc content was analyzed by HPLC and MS as described above.
Results
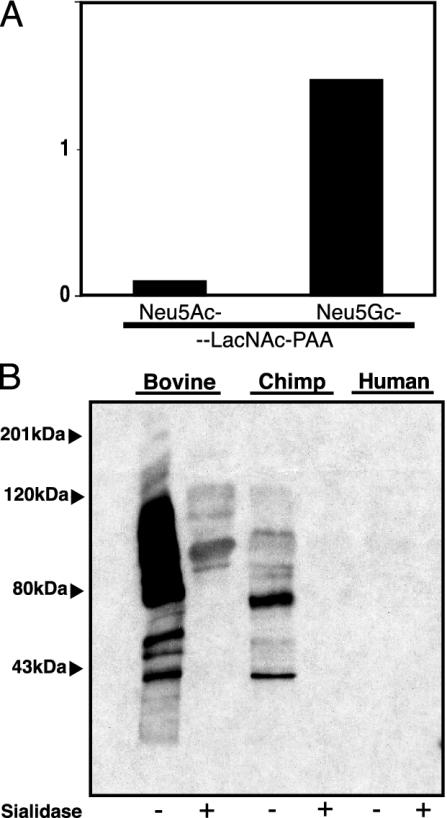
Affinity Purification and Characterization of a Monospecific Antibody Against Neu5Gc. Chickens are the only species other than humans known to generate an immune response to injected Neu5Gc (20, 28–30). A previous attempt at affinity purification of such antibodies used a Neu5Gc-containing glycolipid column, but did not rigorously define specificity (29). We developed a multistep approach to affinity chromatography that optimizes the specificity and avidity of the final preparation (see Fig. 7, which is published as supporting information on the PNAS web site, for details). The antibody preparation that bound to the fetuin column and then rebound to a modified fetuin column reacted with synthetic Neu5Gc-LacNAc-PAA but not with Neu5Ac-LacNAc-PAA, which differ by only a single oxygen atom (Fig. 1A). It also recognized many proteins in Neu5Gc containing bovine and chimpanzee sera, but not in human serum (Fig. 1B, note that chimpanzee proteins are ≈99–100% identical to those of humans) (31). Sialidase pretreatment abrogated reactivity (Fig. 1B). Antibody specificity was confirmed for immunohistochemistry analyses (data not shown), where chimpanzee tissue sections stained intensely and human tissues showed only a limited staining (the latter was later shown to be specific, see below). In addition to the original immunogen (Neu5Gcα2–3Galβ1–4Glc1–1′Ceramide), this preparation recognizes Neu5Gc in either α2–3 or α2–6 linkage to underlying Galβ1–4GlcNAc(LacNAc)-PAA (data not shown). This reactivity was also blocked by chimpanzee, but not human serum (data not shown). Thus, this polyclonal preparation contains antibodies recognizing Neu5Gc on both glycoproteins and glycolipids, regardless of the linkage (α2–3or α2–6) and regardless of the underlying sugar chain. Binding to synthetic PAA-based probes confirms that the nature of the protein or the lipid tail of glycolipids are not important binding determinants.
Fig. 1.
Characterization of specificity of the anti-Neu5Gc antibody. (A) ELISA against Neu5Ac-LacNAc-PAA or Neu5Gc-LacNAc-PAA. Data show the mean of three determinations. (B) Bovine, chimpanzee, or human serum proteins (5 μg each) were either treated or sham-treated with sialidase and separated by SDS/PAGE. Reactivity of the anti-Neu5Gc antibody was analyzed in a Western blot assay.
Antibody Reactivity in Malignant, Fetal, and Normal Human Tissues. Staining with the antibody showed cell type-specific reactivity in adult human tissues. A summary of findings is presented in Table 1, which is published as supporting information on the PNAS web site, and examples are shown in Fig. 2. Breast carcinomas showed reactivity in the majority of tumor cells and some related blood vessels. In the fetus, staining was prominent in epithelial cells and secretions, as well as in the placental villus blood vessels. The overall pattern of staining in 12 normal human tissues can be summarized as prominent in secretory epithelia and associated secretions and present in many blood vessels. Secondary antibody alone with or without normal IgY gave no staining (data not shown). The ability to specifically inhibit staining by chimpanzee serum or porcine mucin varied with individual human tissues (data not shown). However, in comparison to WT mice no clear staining was seen in tissues from CMAH null mice that are expected to be Neu5Gc-deficient (unpublished work). Thus, the variable inhibition by chimpanzee serum or porcine mucin in different human tissues may reflect expression of different types of Neu5Gc-containing epitopes. Of course, we cannot rule out a novel human-specific epitope that is not Neu5Gc and still cross-reacts with this extensively characterized antibody. To be certain of our finding, we turned to MS analyses.
Fig. 2.
Examples of reactivity of anti-Neu5Gc antibody with human tissue sections. Reactivity of the antibody to tissue sections is indicated by the brown staining. (A) Blood vessels in placental villi. (B) Breast carcinoma, tumor cells, and blood vessel. (C) Skin and eccrine glands. (D) Kidney tubules and secretions. For a summary of staining patterns for all tissues studied see Table 1. (Magnification: ×20.)
MS Analysis of Human Tissues Confirms Neu5Gc. Sias were released and purified from human heart, kidney, liver, and spleen glycopeptides and analyzed by DMB-HPLC plus MS (14). A Neu5Gc standard gave signals at m/z 442 and 424, consistent with molecular ions of DMB-derivatized Neu5Gc and its dehydrated form (data not shown). As expected, selective secondary MS on the m/z 442 ion peak gave the m/z 424 dehydrated form (data not shown). MS on putative DMB-derivatized Neu5Gc from human kidney also gave ion peaks at m/z 442 and 424 (Fig. 8, which is published as supporting information on the PNAS web site). Selective secondary MS of the m/z 442 ion gave a single ion peak at m/z 424, confirming the presence of Neu5Gc (Fig. 8). Analysis of glycopeptides and some glycolipid fractions from human heart, liver, and spleen tissues gave similar spectra. MS done in this manner is not truly quantitative. However, we had earlier noted small HPLC peaks from normal adult human tissues eluting in the position expected for Neu5Gc (8). These peaks (now confirmed to be Neu5Gc) represented <1% of total Sias in liver, skin, and kidney, ≈1% in spleen and testes, and ≈2% in the heart (8).
Human Epithelial Cells in Culture Can Metabolically Incorporate Neu5Gc. Although human cells cultured in FCS express cell surface Neu5Gc in small amounts (8, 24), this expression could represent either metabolic incorporation or nonspecific absorption. Human epithelial cells were cultured in human serum until no traces of Neu5Gc derived from FCS could be detected by HPLC (typically ≈4 days). Culture was then continued for 3 days in the absence or presence of varying amounts of free Neu5Gc. HPLC analysis showed increasing incorporation of Neu5Gc into the cells over time, with the highest level reaching 85% of total Sia after incubation in 3 mM Neu5Gc for 3 days (data not shown). Metabolic incorporation of Neu5Gc into glycoproteins was confirmed by Western blotting using the anti-Neu5Gc antibody (Fig. 3). Staining was seen only when Neu5Gc was present in the medium, and reactivity was eliminated by sialidase treatment (further confirming the specificity of the antibody). These data suggested that the small amounts of Neu5Gc in normal human tissues could originate from dietary sources.
Fig. 3.
Incorporation of free Neu5Gc into glycoproteins of cultured human epithelial cells. Western blot analysis of 7 μg of total proteins extracted from Caco-2 cells not fed or fed with Neu5Gc. Proteins were treated or sham-treated with A. ureafaciens sialidase and separated by SDS/PAGE on a 12.5% gel. Reactivity with the anti-Neu5Gc antibody was analyzed in a Western blot assay.
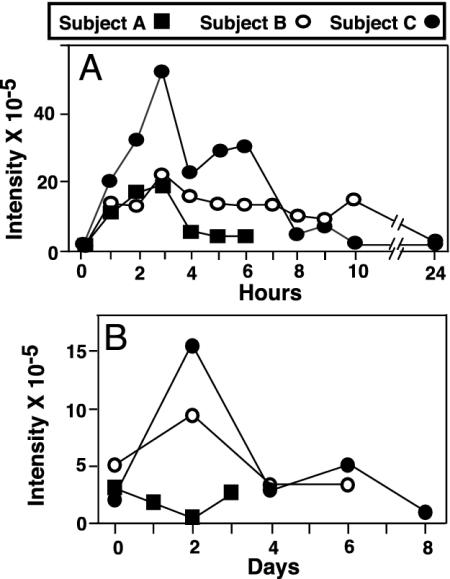
Ingested Neu5Gc Is Absorbed and Excreted by Normal Adult Humans. Purified Neu5Gc was orally administered, and its fate was followed by examination of blood, serum, urine, hair, and saliva. Considering the uncertain risks, three of the authors (A.V., E.M, and P.G.) volunteered as subjects. Initial studies on the first subject indicated no immediate ill effects from ingestion of up to 150 mg of porcine mucin Sias (5% Neu5Ac, 95% Neu5Gc). The other two subjects then also ingested the same amount. There were no changes in vital signs, basic hematology, and serum chemistry values. Free Sias were purified from urine samples and analyzed for Neu5Gc content by DMB-HPLC. Because overlapping fluorescent peaks prevented definitive identification and quantification, HPLC fractions eluting at the time expected for DMB-Neu5Gc were collected and subjected to MS, confirming an increase in ion peaks at m/z 424 and m/z 442. The sum of ion intensities of m/z 424 and m/z 442 at each time point provided an estimate of Neu5Gc quantity. Ingested Neu5Gc was rapidly absorbed and excreted into the urine (Fig. 4A). About 3–6% of the ingested dose was excreted within 4–6 h, with the peak excretion rate at 2–3 h and a return to baseline levels within 24 h. Notably, small amounts of Neu5Gc were also present at baseline.
Fig. 4.
MS detection of urinary excretion and salivary mucin incorporation of ingested Neu5Gc. Sias were purified from the urine (A) and salivary mucins (B)of the three subjects before Neu5Gc ingestion and at various subsequent time points and analyzed by DMB derivatization, HPLC, and MS. The sum of the intensities of ions at m/z 424 and m/z 442 at each time point is plotted for each subject.
Sias could be converted into their corresponding acylmannosamines by sialate:pyruvate-lyases (32), derived from either intestinal cells or gut microbes. Such molecules could then enter cells and be reconverted back to the original Sias (33). To ascertain potential conversion of the ingested Neu5Gc into N-glycolylmannosamine (ManNGc), we looked for this neutral sugar in the urine. Small amounts of ManNGc were present at baseline in one subject (data not shown). There was no obvious increase over time in any subject (data not shown), indicating that the remaining ingested Neu5Gc was not absorbed and excreted as ManNGc. Thus, much of the uptake is apparently occurring as free Neu5Gc.
Ingested Neu5Gc Can Be Incorporated into Newly Synthesized Glycoproteins. Mucins from all baseline saliva samples showed very small amounts of Neu5Gc detectable by MS. An increase 2 days after ingestion occurred in two of three subjects, with a decline to baseline levels by 4 days (Fig. 4B). Although we failed to detect Neu5Gc in serum samples at all time points, small amounts were found in facial hair clippings at baseline for two subjects, with increases after ingestion. Overall the data show uptake and excretion of intact Neu5Gc and low-level metabolic incorporation into newly synthesized glycoconjugates. Although there were no immediate reactions to Neu5Gc ingestion, this does not rule out long-term consequences. Because the basic findings were consistent, we did not risk further studies on ourselves, nor seek additional subjects.
Neu5Gc Is Enriched in Food Sources of Mammalian Origin. Sias have never been detected in plants and are found in large amounts primarily in vertebrates and a few “higher” invertebrates (2, 3, 34). Although several pathogenic microbes can express Neu5Ac, none have been reported to express Neu5Gc. We determined the amounts of Neu5Gc in common dietary sources of animal origin (see Table 2, which is published as supporting information on the PNAS web site). The highest amount was found in lamb, pork, and beef (so-called “red meat”). In contrast, levels were very low or undetectable in poultry and fish. Hen eggs contain primarily Neu5Ac (35). Intermediate amounts were found in bovine milk and milk products, with higher levels in goat and sheep milk products. As summarized in Table 2, U.S. Department of Agriculture-recommended dietary intake would result in substantial intake of Neu5Gc from red meats and moderate amounts from dairy products.
Circulating Anti-Neu5Gc Antibodies Are Present in Most Normal Humans. A sensitive and specific assay for detecting anti-Neu5Gc antibodies in human sera was set up, comparing reactivity against Neu5Gc-PAA and Neu5Ac-PAA, which differ by only one oxygen atom. Anti-Neu5Gc antibodies of the IgA, IgM, and IgG classes were detected in the sera of all three subjects who ingested Neu5Gc at baseline, but no increases occurred after Neu5Gc ingestion (data not shown). Studies of additional normal adults showed that 16/18, 14/18, and 17/18 sera were positive for IgA, IgM, and IgG antibodies, respectively (Fig. 5A). Specificity was confirmed by showing that anti-Neu5Gc reactivity was blocked by chimpanzee serum in a concentration-dependent manner, but not by equivalent amounts of a Neu5Gc antibody-negative human serum (Fig. 5B). The chimpanzee serum used for inhibition was also negative for anti-Neu5Gc antibodies (Fig. 6). Further confirmation came from >70% inhibition of reactivity by free Neu5Gc but not by Neu5Ac (each at 2 mM, data not shown).
Fig. 5.
ELISA detection of anti-Neu5Gc antibodies in normal human sera and demonstration of specificity. (A) Results are plotted as mean background values with PAA-Neu5Ac subtracted from the signal with PAA-Neu5Gc. The mean value for all positive sera in each subclass is represented by the horizontal bar. (B) Two of the human sera identified as medium or high positive for anti-Neu5Gc IgG antibodies to the target Neu5Gc-PAA were tested in the same ELISA with dilutions of chimpanzee serum added to inhibit binding. A human serum with undetectable anti-Neu5Gc antibodies was used as a control.
Fig. 6.
Comparison of levels of anti-Neu5Gc and anti-α-Gal antibodies in normal human sera. Serum samples were collected several months later from the same individuals studied in Fig. 4A. Levels of anti-Neu5Gc antibodies were studied identically, in comparison with parallel wells coated with Galα1–3Galbeta1–4GlcNAc-PAA, instead of Neu5Gc-PAA. The final readout of values was thus directly comparable between the two probes, after subtraction of the background readings with Neu5Ac-PAA. IgG levels were quantified as described.
Some human sera required up to 3–4% chimpanzee serum to obtain complete blockade (Fig. 5B). Because chimpanzee serum glycoproteins carry ≈0.1 nmol of Neu5Gc per μl, the anti-Neu5Gc antibodies must be present at relatively high concentrations in such human sera. Indeed, these antibodies represented up to 0.25% of the total circulating IgG in some subjects (Fig. 6). This falls into the range of the well known xenoreactive antibodies against the nonhuman α-Gal epitope (36, 37). We also compared levels of IgG anti-Neu5Gc and anti-α-Gal antibodies. As expected from prior literature, all of the human sera contained easily detectable anti-α-Gal antibodies. Anti-Neu5Gc levels were highly variable, and there was no direct correlation between the two antibody levels in any given individual. On the other hand, the relative levels of anti-Neu5Gc antibodies between individuals were reproducible on repeat sampling. Four chimpanzee sera showed no anti-Neu5Gc antibodies, but clear evidence of anti-α-Gal antibodies (Fig. 6).
Discussion
Existing data about Neu5Gc in human cells is controversial (16, 18, 19, 21, 22). Even in studies claiming its presence in malignant tissues, the cell type specificity of the presumed Neu5Gc was not defined. We have advanced understanding of this issue by using an affinity-purified polyclonal antibody against Neu5Gc. Our data support prior reports of Neu5Gc in human cancers and extends the finding to normal and fetal human tissues. Of course, despite our extensive efforts to define specificity, we cannot rule out the possibility of an unexpected epitope cross-reactive with this polyclonal antibody. Thus, we confirmed the presence of Neu5Gc in the normal human tissues with MS.
The inactivating mutation of CMAH in humans is genetically irreversible, no human genes have homology to CMAH, and evidence for an alternate biosynthetic pathway is lacking. Although human cells cultured in FCS appear to incorporate Neu5Gc (8, 24), this could be just passive adsorption. For example, glycolipids containing Neu5Gc are lipophilic molecules that can be directly integrated into plasma membranes. Although this could account for some of the Neu5Gc in gut epithelial cells, it cannot explain the enrichment of Neu5Gc in other internal human organs. Here we demonstrate that human cells in culture can metabolically incorporate free Neu5Gc into endogenous glycoproteins. Based on known cellular pathways (13), a possible explanation is that free Neu5Gc enters human cells via pinocytosis to reach the endosomal/lysosomal system. There it could be transported into the cytosolic compartment and get activated to CMP-Neu5Gc, which would eventually enter the Golgi apparatus, where sialytransferases could catalyze transfer of Neu5Gc to newly synthesized glycoconjugates. Notably, all of the transporters and enzymes mentioned above can act on both Neu5Ac and Neu5Gc.
Regardless of the mechanisms of metabolic incorporation, we show that dietary sources could account for the small amount of Neu5Gc in normal adult human tissues. Intact humans absorbed a portion of ingested Neu5Gc, excreted it into the urine, and incorporated a small amount into newly synthesized glycoconjugates. The majority of ingested Neu5Gc was not absorbed, was eliminated from the body by means other than urinary excretion, or was possibly incorporated into tissues not analyzed in this study. These findings differ from a report in rats, where ≈98% of orally administered radioactive Sia was absorbed and excreted unaltered in urine within 6 h (38). This difference could reflect humanspecific mechanisms to exclude the antigenic Neu5Gc either by decreasing absorption or eliminating Neu5Gc via other means. In this regard, we noted selective staining of the epithelia and the associated secretions in normal tissues (i.e., cells with a high turnover rate, and which secrete sialoglyconjugates). The more prominent enrichment of Neu5Gc in carcinomas and fetuses could be caused by higher uptake by these rapidly growing tissues. The contrasting absence of Neu5Gc from plasma proteins and blood cells could be because the cells of origin (hepatocytes, plasma cells, and bone marrow cells) have a robust ability to generate their own Neu5Ac from endogenous pathways, which would block competition by exogenous Neu5Gc.
We found that most normal humans have circulating anti-Neu5Gc antibodies. While this article was in preparation, another group independently reported a high prevalence of anti-Neu5Gc antibodies in normal human sera, using a different approach (39). The findings of both studies stand in contrast to previous reports of the prevalence of anti-Neu5Gc antibodies in normal humans, ranging only from 0–10% for the IgG class to 3.8–13.8% for the IgM class (40–42). This disparity can be explained by the greater sensitivity and lower background of the assays we used, the use of an optimal control target (identical except for Neu5Ac in place of Neu5Gc), and the checks for specificity (blockade by chimpanzee but not human serum, or by free Neu5Gc but not free Neu5Ac).
The anti-Neu5Gc antibodies could be a response to dietary Neu5Gc and/or to endogenously incorporated Neu5Gc. Although most normal human adults had anti-Neu5Gc antibodies, one-time ingestion of a large amount of Neu5Gc did not enhance baseline levels. This finding may reflect incorporation of the Neu5Gc into immune privileged cell types, rapid elimination of Neu5Gc via excretion by epithelial cells, or a preexisting partial tolerization to Neu5Gc. Another possibility is that induction of an immune response necessitates Neu5Gc accumulation and/or exposure over long time period. Other variables are the extent of maternal-fetal transfer of Neu5Gc and early childhood exposure to Neu5Gc in bovine milk or vaccines. Population studies are needed to pursue such issues.
Some humans had circulating anti-Neu5Gc IgG levels in the range seen for xenoreactive antibodies against the α-Gal epitope that is deficient in all Old World primates, including humans (36, 37). Thus, anti-Neu5Gc antibodies comprise another potential barrier to xenotransplantation, especially from species such as the pig, whose organs are rich in Neu5Gc. They also pose a potential limitation for the use of glycosylated therapeutic agents generated in the milk of animals or in nonhuman cells. There are differences between the two types of antibodies. First, although anti-α-Gal antibodies occur at relatively high levels in all humans, anti-Neu5Gc antibody levels vary greatly. Second, unlike Neu5Gc, the α-Gal epitope itself is completely absent from normal humans and cannot be metabolically incorporated from dietary sources. Third, the anti-α-Gal response is apparently generated by postnatal exposure to exogenous antigens. In contrast, the anti-Neu5Gc response could be caused by exogenous antigens or endogenously incorporated molecules.
The remainder of this discussion contains speculation that is either directly testable or epidemiologically approachable. Consumption of red meat (the richest source of Neu5Gc) is associated with diseases such as cancer (43–48) and ischemic heart disease (44, 45, 49). In contrast, vegetarianism decreases the risk of cancer (44, 46) and heart disease (44, 45, 49). Although saturated fat in meat and dairy products is the usual explanation, population studies comparing anti-Neu5Gc antibodies and/or body burden of Neu5Gc with cancer and ischemic heart disease incidence may be worthwhile.
Some other diseases such as hepatitis, liver cirrhosis, infectious mononucleosis, rheumatoid arthritis, syphilis, and lepromatous leprosy are claimed to be associated with “Hanganutziu-Deicher” heterophile anti-Neu5Gc antibodies (40–42). Such antibodies could play a role in initiation or progression or simply arise from immune dysregulation that allows preexisting memory B cells to expand. Alternatively, some of these diseases involve proliferation of vascularized tissues that could incorporate more dietary Neu5Gc, boosting a baseline immune response. The small amounts of Neu5Gc in normal tissues also raise the possibility that anti-Neu5Gc antibodies are involved in autoimmunity. In this regard, it is interesting that vegetarian diet has been suggested to improve rheumatoid arthritis (50).
The CMAH mutation occurred ≈2.5 million to 3 million years ago (14), before the first evidence for meat eating in the subsequently emerging Homo lineage (51). Of course, most diseases potentially associated with dietary Neu5Gc and/or Neu5Gc antibodies have an onset beyond the average age of reproduction, when natural selection is weak or nonexistent (52). Thus, there may not have been much selection initially against Neu5Gc intake and incorporation. However, in this era of increased longevity, the long-term consequences of constantly challenging our systems with this antigenic sugar and the implications of having anti-Neu5Gc antibodies in the circulation are provocative and should be further investigated.
Supplementary Material
Acknowledgments
We thank Takashi Angata, Justin Sonnenburg, and Brad Hayes for helpful discussions, valuable reagents, and analyses. This work was supported by U.S. Public Health Service Grant R01GM323373 and the G. Harold and Leila Y. Mathers Charitable Foundation. Some human studies were done in the University of California, San Diego General Clinical Research Center (supported by National Institutes of Health Grant M01RR00827). M.B. was supported by the French Government.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Sia, sialic acid; Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; CMAH, CMP-Neu5Ac hydroxylase; DMB, 1,2-diamino-4,5-methylene dioxybenzene; α-Gal, α-galactose; PAA, polyacrylamide.
References
- 1.Gottschalk, A. (1960) The Chemistry and Biology of Sialic Acids (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Rosenberg, A. & Schengrund, C. (1976) Biology of Sialic Acids (Plenum, New York).
- 3.Schauer, R. (1982) Adv. Carbohydr. Chem. Biochem. 40, 131–234. [DOI] [PubMed] [Google Scholar]
- 4.Angata, T. & Varki, A. (2002) Chem. Rev. 102, 439–470. [DOI] [PubMed] [Google Scholar]
- 5.Shaw, L. & Schauer, R. (1988) Biol. Chem. Hoppe-Seyler 369, 477–486. [DOI] [PubMed] [Google Scholar]
- 6.Kozutsumi, Y., Kawano, T., Yamakawa, T. & Suzuki, A. (1990) J. Biochem. (Tokyo) 108, 704–706. [DOI] [PubMed] [Google Scholar]
- 7.Muchmore, E. A., Milewski, M., Varki, A. & Diaz, S. (1989) J. Biol. Chem. 264, 20216–20223. [PubMed] [Google Scholar]
- 8.Muchmore, E. A., Diaz, S. & Varki, A. (1998) Am. J. Phys. Anthropol. 107, 187–198. [DOI] [PubMed] [Google Scholar]
- 9.Higashi, H., Naiki, M., Matuo, S. & Okouchi, K. (1977) Biochem. Biophys. Res. Commun. 79, 388–395. [DOI] [PubMed] [Google Scholar]
- 10.Merrick, J. M., Zadarlik, K. & Milgrom, F. (1978) Int. Arch. Allergy Appl. Immunol. 57, 477–480. [DOI] [PubMed] [Google Scholar]
- 11.Chou, H. H., Takematsu, H., Diaz, S., Iber, J., Nickerson, E., Wright, K. L., Muchmore, E. A., Nelson, D. L., Warren, S. T. & Varki, A. (1998) Proc. Natl. Acad. Sci. USA 95, 11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irie, A., Koyama, S., Kozutsumi, Y., Kawasaki, T. & Suzuki, A. (1998) J. Biol. Chem. 273, 15866–15871. [DOI] [PubMed] [Google Scholar]
- 13.Varki, A. (2002) Yearbook Phys. Anthropol. 44, 54–69. [Google Scholar]
- 14.Chou, H. H., Hayakawa, T., Diaz, S., Krings, M., Indriati, E., Leakey, M., Paabo, S., Satta, Y., Takahata, N. & Varki, A. (2002) Proc. Natl. Acad. Sci. USA 99, 11736–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirabayashi, Y., Kasakura, H., Matsumoto, M., Higashi, H., Kato, S., Kasai, N. & Naiki, M. (1987) Jpn. J. Cancer Res. 78, 251–260. [PubMed] [Google Scholar]
- 16.Higashi, H., Hirabayashi, Y., Fukui, Y., Naiki, M., Matsumoto, M., Ueda, S. & Kato, S. (1985) Cancer Res. 45, 3796–3802. [PubMed] [Google Scholar]
- 17.Miyoshi, I., Higashi, H., Hirabayashi, Y., Kato, S. & Naiki, M. (1986) Mol. Immunol. 23, 631–638. [DOI] [PubMed] [Google Scholar]
- 18.Marquina, G., Waki, H., Fernandez, L. E., Kon, K., Carr, A., Valiente, O., Perez, R. & Ando, S. (1996) Cancer Res. 56, 5165–5171. [PubMed] [Google Scholar]
- 19.Devine, P. L., Clark, B. A., Birrell, G. W., Layton, G. T., Ward, B. G., Alewood, P. F. & McKenzie, I. F. C. (1991) Cancer Res. 51, 5826–5836. [PubMed] [Google Scholar]
- 20.Kawachi, S., Saida, T., Uhara, H., Uemura, K., Taketomi, T. & Kano, K. (1988) Int. Arch. Allergy Appl. Immunol. 85, 381–383. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa, K., Yamaguchi, H., Oettgen, H. F., Old, L. J. & Lloyd, K. O. (1988) J. Biol. Chem. 263, 18507–18512. [PubMed] [Google Scholar]
- 22.Vázquez, A. M., Alfonso, M., Lanne, B., Karlsson, K. A., Carr, A., Barroso, O., Fernández, L. E., Rengifo, E., Lanio, M. E., Alvarez, C., et al. (1995) Hybridoma 14, 551–556. [DOI] [PubMed] [Google Scholar]
- 23.Kawai, T., Kato, A., Higashi, H., Kato, S. & Naiki, M. (1991) Cancer Res. 51, 1242–1246. [PubMed] [Google Scholar]
- 24.Oetke, C., Hinderlich, S., Brossmer, R., Reutter, W., Pawlita, M. & Keppler, O. T. (2001) Eur. J. Biochem. 268, 4553–4561. [DOI] [PubMed] [Google Scholar]
- 25.Varki, A. & Diaz, S. (1984) Anal. Biochem. 137, 236–247. [DOI] [PubMed] [Google Scholar]
- 26.Klein, A., Diaz, S., Ferreira, I., Lamblin, G., Roussel, P. & Manzi, A. E. (1997) Glycobiology 7, 421–432. [DOI] [PubMed] [Google Scholar]
- 27.Bjorndal, H., Lindberg, B., Pilotti, A. & Svensson, S. (1967) Carbohydr. Res. 5, 433–440 [Google Scholar]
- 28.Fujii, Y., Higashi, H., Ikuta, K., Kato, S. & Naiki, M. (1982) Mol. Immunol. 19, 87–94. [DOI] [PubMed] [Google Scholar]
- 29.Hirabayashi, Y., Suzuki, T., Suzuki, Y., Taki, T., Matsumoto, M., Higashi, H. & Kato, S. (1983) J. Biochem. (Tokyo) 94, 327–330. [DOI] [PubMed] [Google Scholar]
- 30.Asaoka, H., Nishinaka, S., Wakamiya, N., Matsuda, H. & Murata, M. (1992) Immunol. Lett. 32, 91–96. [DOI] [PubMed] [Google Scholar]
- 31.Goodman, M. (1999) Am. J. Hum. Genet. 64, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schauer, R., Sommer, U., Krüger, D., Van, U. H. & Traving, C. (1999) Biosci. Rep. 19, 373–383. [DOI] [PubMed] [Google Scholar]
- 33.Kayser, H., Zeitler, R., Kannicht, C., Grunow, D., Nuck, R. & Reutter, W. (1992) J. Biol. Chem. 267, 16934–16938. [PubMed] [Google Scholar]
- 34.Warren, L. (1963) Comp. Biochem. Physiol. 10, 153–171. [DOI] [PubMed] [Google Scholar]
- 35.Juneja, L. R., Koketsu, M., Nishimoto, K., Kim, M., Yamamoto, T. & Itoh, T. (1991) Carbohydr. Res. 214, 179–186. [DOI] [PubMed] [Google Scholar]
- 36.Galili, U. (1993) Immunol. Today 14, 480–482. [DOI] [PubMed] [Google Scholar]
- 37.Joziasse, D. H. & Oriol, R. (1999) Biochim. Biophys. Acta Mol. Basis Dis. 1455, 403–418. [DOI] [PubMed] [Google Scholar]
- 38.Nohle, U., Beau, J. M. & Schauer, R. (1982) Eur. J. Biochem. 126, 543–548. [DOI] [PubMed] [Google Scholar]
- 39.Zhu, A. & Hurst, R. (2002) Xenotransplantation 9, 376–381. [DOI] [PubMed] [Google Scholar]
- 40.Morito, T., Nishimaki, T., Masaki, M., Yoshida, H., Kasukawa, R., Nakarai, H. & Kano, K. (1986) Int. Arch. Allergy Appl. Immunol. 81, 204–208. [DOI] [PubMed] [Google Scholar]
- 41.Morito, T., Kano, K. & Milgrom, F. (1982) J. Immunol. 129, 2524–2528. [PubMed] [Google Scholar]
- 42.Higashihara, T., Takeshima, T., Anzai, M., Tomioka, M., Matsumoto, K., Nishida, K., Kitamura, Y., Okinaga, K. & Naiki, M. (1991) Int. Arch Allergy Appl. Immunol. 95, 231–235. [DOI] [PubMed] [Google Scholar]
- 43.Rose, D. P., Boyar, A. P. & Wynder, E. L. (1986) Cancer 58, 2363–2371. [DOI] [PubMed] [Google Scholar]
- 44.Fraser, G. E. (1999) Am. J. Clin. Nutr. 70, 532S–538S. [DOI] [PubMed] [Google Scholar]
- 45.Key, T. J., Fraser, G. E., Thorogood, M., Appleby, P. N., Beral, V., Reeves, G., Burr, M. L., Chang-Claude, J., Frentzel-Beyme, R., Kuzma, J. W., et al. (1999) Am. J. Clin. Nutr. 70, 516S–524S. [DOI] [PubMed] [Google Scholar]
- 46.Phillips, R. L., Garfinkel, L., Kuzma, J. W., Beeson, W. L., Lotz, T. & Brin, B. (1980) J. Natl. Cancer Inst. 65, 1097–1107. [PubMed] [Google Scholar]
- 47.Kolonel, L. N. (2001) Epidemiol. Rev. 23, 72–81. [DOI] [PubMed] [Google Scholar]
- 48.Willett, W. C. (2000) Oncologist 5, 393–404. [DOI] [PubMed] [Google Scholar]
- 49.Snowdon, D. A., Phillips, R. L. & Fraser, G. E. (1984) Prev. Med. 13, 490–500. [DOI] [PubMed] [Google Scholar]
- 50.Muller, H., De Toledo, F. W. & Resch, K. L. (2002) Scand. J. Rheumatol. 30, 1–10. [DOI] [PubMed] [Google Scholar]
- 51.Wood, B. & Collard, M. (1999) Science 284, 65–66. [DOI] [PubMed] [Google Scholar]
- 52.Hamilton, W. D. (1966) J. Theor. Biol. 12, 12–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.