Abstract
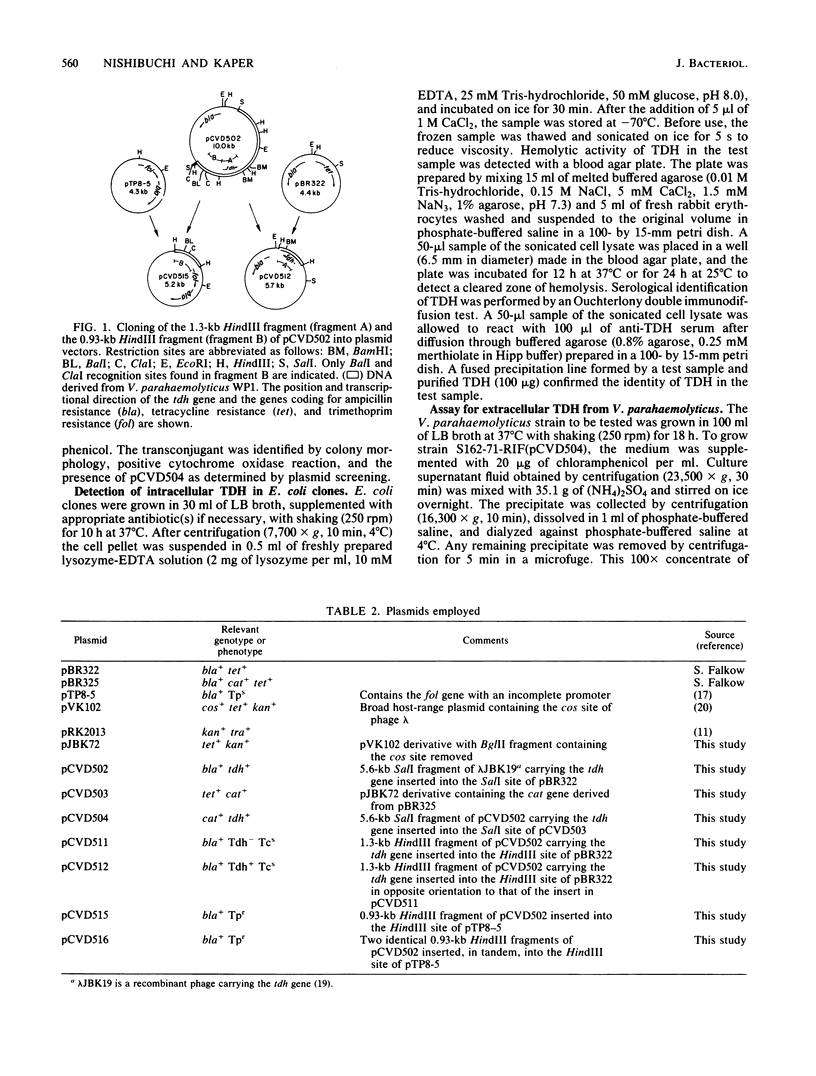
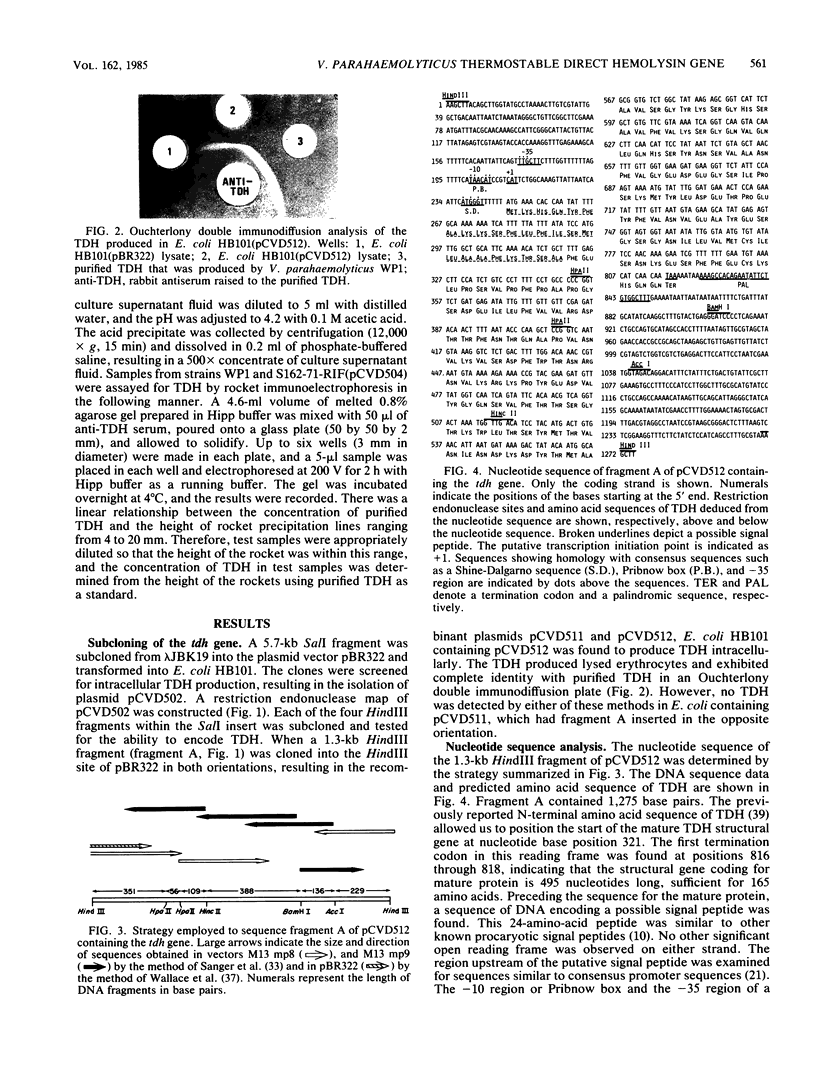
The gene encoding the thermostable direct hemolysin of Vibrio parahaemolyticus was characterized. This gene (designated tdh) was subcloned into pBR322 in Escherichia coli, and the functional tdh gene was localized to a 1.3-kilobase HindIII fragment. This fragment was sequenced, and the structural gene was found to encode a mature protein of 165 amino acid residues. The mature protein sequence was preceded by a putative signal peptide sequence of 24 amino acids. A putative tdh promoter, determined by its similarity to concensus sequences, was not functional in E. coli. However, a promoter that was functional in E. coli was shown to exist further upstream by use of a promoter probe plasmid. A 5.7-kilobase SalI fragment containing the structural gene and both potential promoters was cloned into a broad-host-range plasmid and mobilized into a Kanagawa phenomenon-negative V. parahaemolyticus strain. In contrast to E. coli, where the hemolysin was detected only in cell lysates, introduction of the cloned gene into V. parahaemolyticus resulted in the production of extracellular hemolysin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Takanami M., Ohtsuka E., Taniyama Y., Marumoto R., Sato H., Ikehara M. Essential structure of E. coli promoter: effect of spacer length between the two consensus sequences on promoter function. Nucleic Acids Res. 1983 Sep 10;11(17):5855–5864. doi: 10.1093/nar/11.17.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Chun D., Chung J. K., Tak R., Seol S. Y. Nature of the Kanagawa phenomenon of Vibrio parahaemolyticus. Infect Immun. 1975 Jul;12(1):81–87. doi: 10.1128/iai.12.1.81-87.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Milazzo J. P., Sciaky D., Roberts R. J. Computer programs for the assembly of DNA sequences. Nucleic Acids Res. 1979 Sep 25;7(2):529–545. doi: 10.1093/nar/7.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Chearskul S., Takeda Y., Miwatani T. Immunological methods for detection of Kanagawa phenomenon of Vibrio parahaemolyticus. J Clin Microbiol. 1980 Jun;11(6):600–603. doi: 10.1128/jcm.11.6.600-603.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Takeda Y., Miwatani T., Nakahara N. Failure of antisera to thermostable direct hemolysin and cholera enterotoxin to prevent accumulation of fluid caused by Vibrio parahaemolyticus. J Infect Dis. 1983 Apr;147(4):779–779. doi: 10.1093/infdis/147.4.779. [DOI] [PubMed] [Google Scholar]
- Iwakura M., Shimura Y., Tsuda K. Construction of plasmid vectors for cloning of promoters using the dihydrofolate reductase gene of Escherichia coli K-12. J Biochem. 1983 Mar;93(3):927–930. doi: 10.1093/jb/93.3.927. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Campen R. K., Seidler R. J., Baldini M. M., Falkow S. Cloning of the thermostable direct or Kanagawa phenomenon-associated hemolysin of Vibrio parahaemolyticus. Infect Immun. 1984 Jul;45(1):290–292. doi: 10.1128/iai.45.1.290-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Kato T., Obara Y., Akiyama S., Takizawa K., Yamai S. In vitro hemolytic characteristic of Vibrio parahaemolyticus: its close correlation with human pathogenicity. J Bacteriol. 1969 Nov;100(2):1147–1149. doi: 10.1128/jb.100.2.1147-1149.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Obara Y., Nikkawa T., Yamai S., Kato T., Yamada Y., Ohashi M. Simplified purification and biophysicochemical characteristics of Kanagawa phenomenon-associated hemolysin of Vibrio parahaemolyticus. Infect Immun. 1980 May;28(2):567–576. doi: 10.1128/iai.28.2.567-576.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K., Kato T., Obara Y., Yamai S. Studies on the enteropathogenic, facultatively halophilic bacterium, Vibrio parahaemolyticus. 3. Enteropathogenicity. Jpn J Med Sci Biol. 1968 Oct;21(5):325–331. doi: 10.7883/yoken1952.21.325. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Matsuzaki A., Miwatani T. Purification and characterization of thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1973 Nov;8(5):775–780. doi: 10.1128/iai.8.5.775-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y. Thermostable direct hemolysin of Vibrio parahaemolyticus. Pharmacol Ther. 1982;19(1):123–146. doi: 10.1016/0163-7258(82)90044-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- West R. W., Jr, Rodriguez R. L. Construction and characterization of E. coli promoter-probe plasmid vectors. III. pBR322 derivatives with deletions in the tetracycline resistance promoter region. Gene. 1982 Dec;20(2):291–304. doi: 10.1016/0378-1119(82)90047-6. [DOI] [PubMed] [Google Scholar]



