Abstract
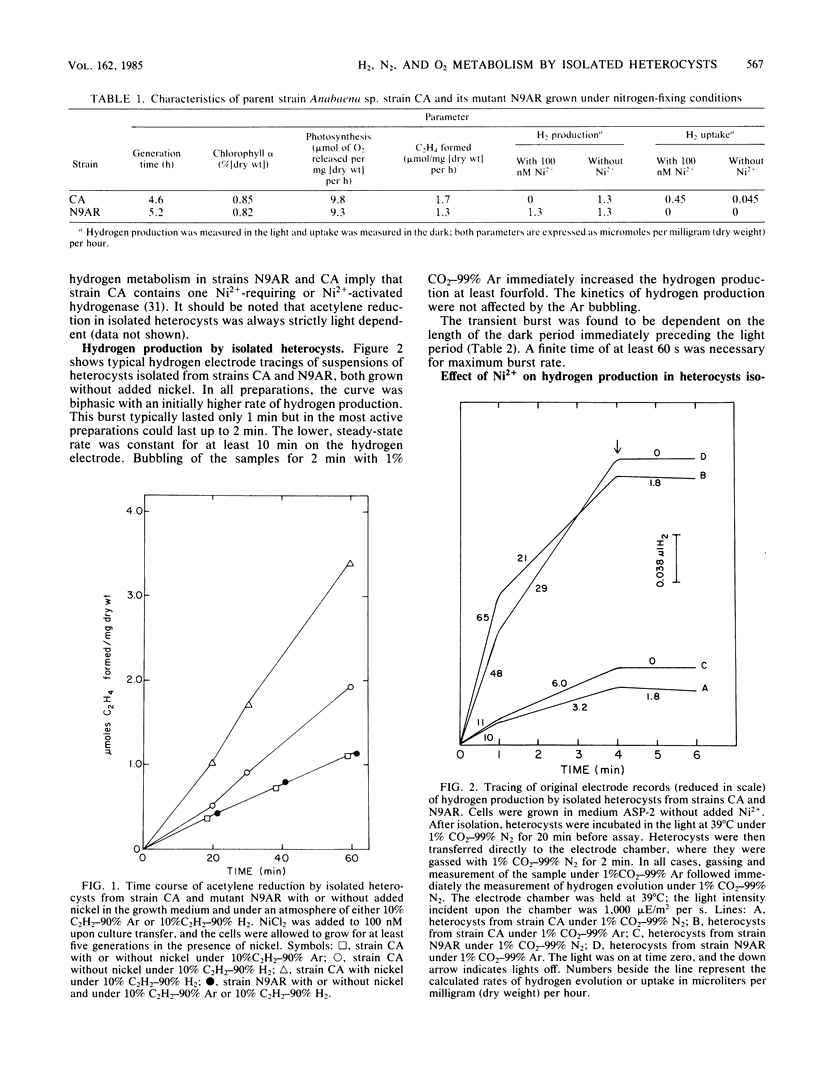
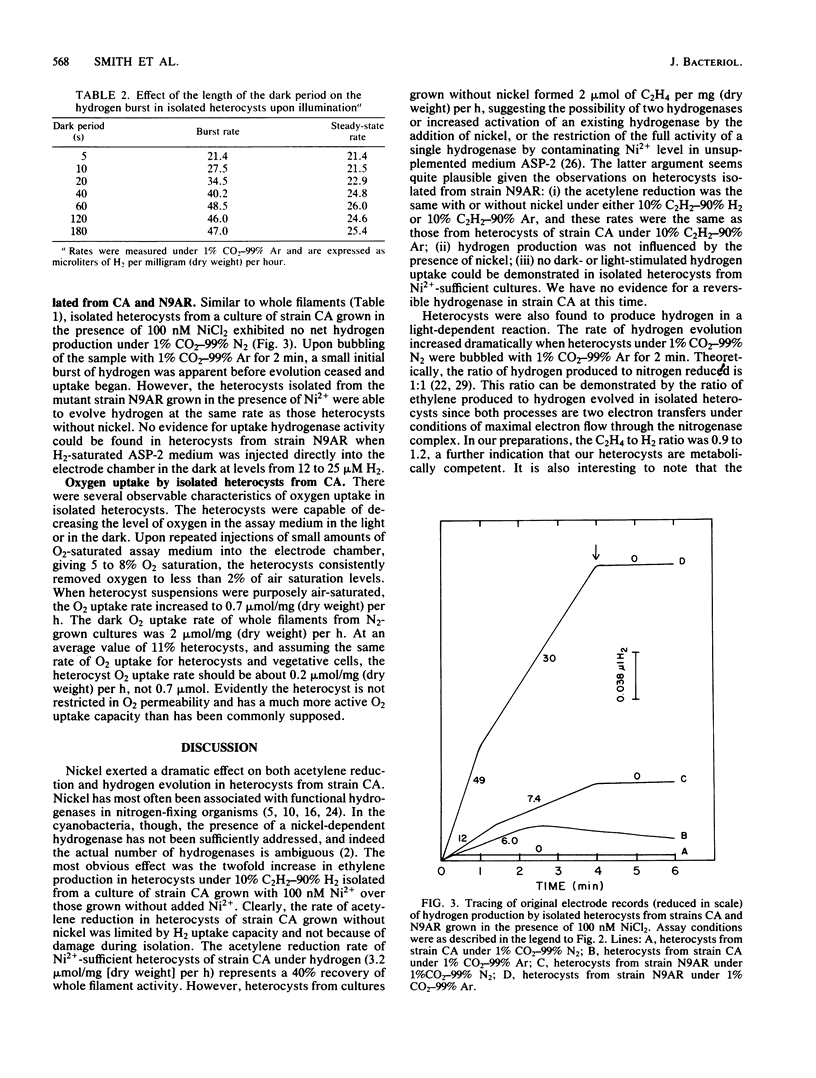
Metabolically active heterocysts isolated from wild-type Anabaena sp. strain CA showed high rates of light-dependent acetylene reduction and hydrogen evolution. These rates were similar to those previously reported in heterocysts isolated from the mutant Anabaena sp. strain CA-V possessing fragile vegetative cell walls. Hydrogen production was observed with isolated heterocysts. The ratio of C2H4 to H2 produced ranged from 0.9 to 1.2, and H2 production exhibited unique biphasic kinetics consisting of a 1 to 2-min burst of hydrogen evolution followed by a lower, steady-state rate of hydrogen production. This burst was found to be dependent upon the length of the dark period immediately preceding illumination and may be related to dark-to-light ATP transients. The presence of 100 nM NiCl2 in the growth medium exerted an effect on both acetylene reduction and hydrogen evolution in the isolated heterocysts from strain CA. H2-stimulated acetylene reduction was increased from 2.0 to 3.2 mumol of C2H4 per mg (dry weight) per h, and net hydrogen production was abolished. A phenotypic Hup- mutant (N9AR) of Anabaena sp. strain CA was isolated which did not respond to nickel. In isolated heterocysts from N9AR, ethylene production rates were the same under both 10% C2H2-90% Ar and 10% C2H2-90% H2 with or without added nickel, and net hydrogen evolution was not affected by the presence of 100 nM Ni2+. Isolated heterocysts from strain CA were shown to have a persistent oxygen uptake of 0.7 mumol of O2 per mg (dry weight) per h, 35% of the rate of whole filaments, at air saturating O2 levels, indicating that O2 impermeability is not a requirement for active heterocysts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardemil L., Wolk C. P. The polysaccharides from heterocyst and spore envelopes of a blue-green alga. Methylation analysis and structure of the backbones. J Biol Chem. 1976 May 25;251(10):2967–2975. [PubMed] [Google Scholar]
- Daday A., Platz R. A., Smith G. D. Anaerobic and aerobic hydrogen gas formation by the blue-green alga Anabaena cylindrica. Appl Environ Microbiol. 1977 Nov;34(5):478–483. doi: 10.1128/aem.34.5.478-483.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. Photostimulation of nitrogen fixation in Anabaena cylindrica. Biochim Biophys Acta. 1970 Sep 1;216(2):353–356. doi: 10.1016/0005-2728(70)90226-4. [DOI] [PubMed] [Google Scholar]
- Fay P., Walsby A. E. Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature. 1966 Jan 1;209(5018):94–95. doi: 10.1038/209094a0. [DOI] [PubMed] [Google Scholar]
- Friedrich C. G., Schneider K., Friedrich B. Nickel in the catalytically active hydrogenase of Alcaligenes eutrophus. J Bacteriol. 1982 Oct;152(1):42–48. doi: 10.1128/jb.152.1.42-48.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto J. W., Tabita F. R., Van Baalen C. Mutants of Anabaena strain CA altered in their ability to grow under nitrogen-fixing conditions. J Bacteriol. 1979 Nov;140(2):327–332. doi: 10.1128/jb.140.2.327-332.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Hind G. Flash spectroscopic characterization of photosynthetic electron transport in isolated heterocysts. Arch Biochem Biophys. 1983 Jul 1;224(1):272–282. doi: 10.1016/0003-9861(83)90210-2. [DOI] [PubMed] [Google Scholar]
- Huynh B. H., Czechowski M. H., Krüger H. J., DerVartanian D. V., Peck H. D., Jr, LeGall J. Desulfovibrio vulgaris hydrogenase: a nonheme iron enzyme lacking nickel that exhibits anomalous EPR and Mössbauer spectra. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3728–3732. doi: 10.1073/pnas.81.12.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Bishop N. I. Simultaneous measurement of oxygen and hydrogen exchange from the blue-green alga anabaena. Plant Physiol. 1976 Apr;57(4):659–665. doi: 10.1104/pp.57.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Tabita F. R., Van Baalen C. High endogenous nitrogenase activity in isolated heterocysts of Anabaena sp. strain CA after nitrogen starvation. J Bacteriol. 1983 Aug;155(2):493–497. doi: 10.1128/jb.155.2.493-497.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F. B., Burris R. H. A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science. 1984 Jun 8;224(4653):1095–1097. doi: 10.1126/science.6585956. [DOI] [PubMed] [Google Scholar]
- Stewart W. D. Some aspects of structure and function in N2-fixing cyanobacteria. Annu Rev Microbiol. 1980;34:497–536. doi: 10.1146/annurev.mi.34.100180.002433. [DOI] [PubMed] [Google Scholar]
- Wang R., Healey F. P., Myers J. Amperometric measurement of hydrogen evolution in chlamydomonas. Plant Physiol. 1971 Jul;48(1):108–110. doi: 10.1104/pp.48.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. K., Haskell J. B., Tabita F. R., Van Baalen C. Aerobic hydrogen production by the heterocystous cyanobacteria Anabaena spp. strains CA and 1F. J Bacteriol. 1983 Dec;156(3):1118–1122. doi: 10.1128/jb.156.3.1118-1122.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


