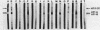Abstract
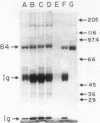
A locus unlinked to either katE or katF that affected catalase levels in Escherichia coli was identified and localized between metB and ppc at 89.2 min on the genome. The locus was named katG. Mutations in katG which prevented the formation of both isoenzyme forms of the bifunctional catalase-peroxidase HPI were created both by nitrosoguanidine and by transposon Tn10 insertions. All katG+ recombinants and transductants contained both HPI isoenzymes. Despite the common feature of little or no catalase activity in four of the catalase-deficient strains, subtle differences in the phenotypes of each strain resulted from the different katG mutations. All three mutants caused by nitrosoguanidine produced a protein with little or no catalase activity but with the same subunit molecular weight and with similar antigenic properties to HPI, implying the presence of missense mutations rather than nonsense mutations in each strain. Indeed one mutant produced an HPI-like protein that retained peroxidase activity, whereas the HPI-like protein in a second mutant exhibited no catalase or peroxidase activity. The third mutant responded to ascorbate induction with the synthesis of near normal catalase levels, suggesting a regulatory defect. The Tn10 insertion mutant produced no catalase and no protein that was antigenically similar to HPI.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne A., Fridovich I. Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J Biol Chem. 1979 May 25;254(10):4245–4252. [PubMed] [Google Scholar]
- Claiborne A., Malinowski D. P., Fridovich I. Purification and characterization of hydroperoxidase II of Escherichia coli B. J Biol Chem. 1979 Nov 25;254(22):11664–11668. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. The gene for ribosomal protein L31, rpmE, is located at 88.5 minutes on the Escherichia coli chromosomal linkage map. J Bacteriol. 1981 Oct;148(1):379–382. doi: 10.1128/jb.148.1.379-382.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Lundblad V., Hanley-Way S., Halling S. M., Kleckner N. Three Tn10-associated excision events: relationship to transposition and role of direct and inverted repeats. Cell. 1981 Jan;23(1):215–227. doi: 10.1016/0092-8674(81)90286-5. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of catalase and peroxidase in Escherichia coli. J Biol Chem. 1978 Sep 25;253(18):6445–6420. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine S. A. Isolation and characterization of catalase deficient mutants of Salmonella typhimurium. Mol Gen Genet. 1977 Jan 18;150(2):205–209. doi: 10.1007/BF00695400. [DOI] [PubMed] [Google Scholar]
- Loewen P. C. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J Bacteriol. 1984 Feb;157(2):622–626. doi: 10.1128/jb.157.2.622-626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1984 Nov;160(2):668–675. doi: 10.1128/jb.160.2.668-675.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L., Klassen G. R., Weiner J. H. Identification and physical characterization of a Col E1 hybrid plasmid containing a catalase gene of Escherichia coli. Can J Biochem Cell Biol. 1983 Dec;61(12):1315–1321. doi: 10.1139/o83-168. [DOI] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Suppressor of phosphofructokinase mutations of Escherichia coli. J Bacteriol. 1972 Oct;112(1):183–187. doi: 10.1128/jb.112.1.183-187.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Kuwano M. Mutation affecting the thermolability of the 50S ribosomal subunit in Escherichia coli. J Bacteriol. 1978 May;134(2):677–679. doi: 10.1128/jb.134.2.677-679.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M. L. The role of adenosine 3',5'-cyclic monophosphate in the growth of bacteriophage lambda. Virology. 1972 Aug;49(2):605–609. doi: 10.1016/0042-6822(72)90513-2. [DOI] [PubMed] [Google Scholar]
- Richter H. E., Loewen P. C. Induction of catalase in Escherichia coli by ascorbic acid involves hydrogen peroxide. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1039–1046. doi: 10.1016/0006-291x(81)91928-8. [DOI] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Rorth M., Jensen P. K. Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta. 1967 May 16;139(1):171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipkas D., Riley M. Proposal concerning mechanism of evolution of the genome of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1354–1358. doi: 10.1073/pnas.72.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]