Abstract
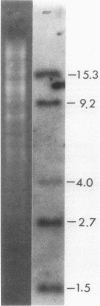
Two clones which contained nif DNA were isolated from a clone bank of total EcoRI-digested Azotobacter vinelandii DNA. The clones carrying the recombinant plasmids were identified by use of the 32P-labeled 6.2-kilobase (kb) nif insert from pSA30 (which contains the Klebsiella pneumoniae nifK, nifD, and nifH genes) as a hybridization probe. Hybridization analysis with fragments derived from the nif insert of pSA30 showed that the 2.6-kb insert from one of the plasmids (pLB1) contains nifK whereas the 1.4-kb insert from the other plasmid (pLB3) contains nifD. Marker rescue tests using genetic transformation indicated that the 2.6-kb A. vinelandii nif fragment contains the wild-type alleles for the nif-6 and nif-38 mutations carried by Nif- strains UW6 and UW38. The 1.4-kb insert contains the wild-type allele for the nif-10 mutation carried by Nif- strain UW10.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. E., Brill W. J. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J Bacteriol. 1977 May;130(2):954–956. doi: 10.1128/jb.130.2.954-956.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Gordon J. K., Shah V. K., Brill W. J. Transformation of nitrogen fixation genes in Azotobacter. Basic Life Sci. 1977;9:67–80. doi: 10.1007/978-1-4684-0880-5_6. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cannon F. C., Riedel G. E., Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterized. Mol Gen Genet. 1979 Jul 2;174(1):59–66. doi: 10.1007/BF00433306. [DOI] [PubMed] [Google Scholar]
- DE WITT C. W., ROWE J. A. N,O-Diacetylneuraminic acid and N-acetylneuraminic acid in Escherichia coli. Nature. 1959 Aug 1;184(Suppl 6):381–382. doi: 10.1038/184381b0. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M., Phadnis S. H., Das H. K. Construction of a gene library from the nitrogen-fixing aerobe Azotobacter vinelandii. Gene. 1983 Nov;25(2-3):355–360. doi: 10.1016/0378-1119(83)90240-8. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Bott K. F. Restriction enzyme analysis of Bacillus subtilis ribosomal ribonucleic acid genes. J Bacteriol. 1979 Oct;140(1):99–105. doi: 10.1128/jb.140.1.99-105.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V. Rapid and simple removal of contaminating RNA from plasmid DNA without the use of RNase. Anal Biochem. 1981 May 1;113(1):34–42. doi: 10.1016/0003-2697(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Physiological factors affecting transformation of Azotobacter vinelandii. J Bacteriol. 1976 Mar;125(3):1080–1087. doi: 10.1128/jb.125.3.1080-1087.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979 Sep;139(3):1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Bott K. F., Newbold J. E. Two-dimensional restriction analysis of the Bacillus subtilis genome: gene purification and ribosomal ribonucleic acid gene organization. J Bacteriol. 1977 Jan;129(1):492–500. doi: 10.1128/jb.129.1.492-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. E., Ausubel F. M., Cannon F. C. Physical map of chromosomal nitrogen fixation (nif) genes of Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2866–2870. doi: 10.1073/pnas.76.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]