Abstract
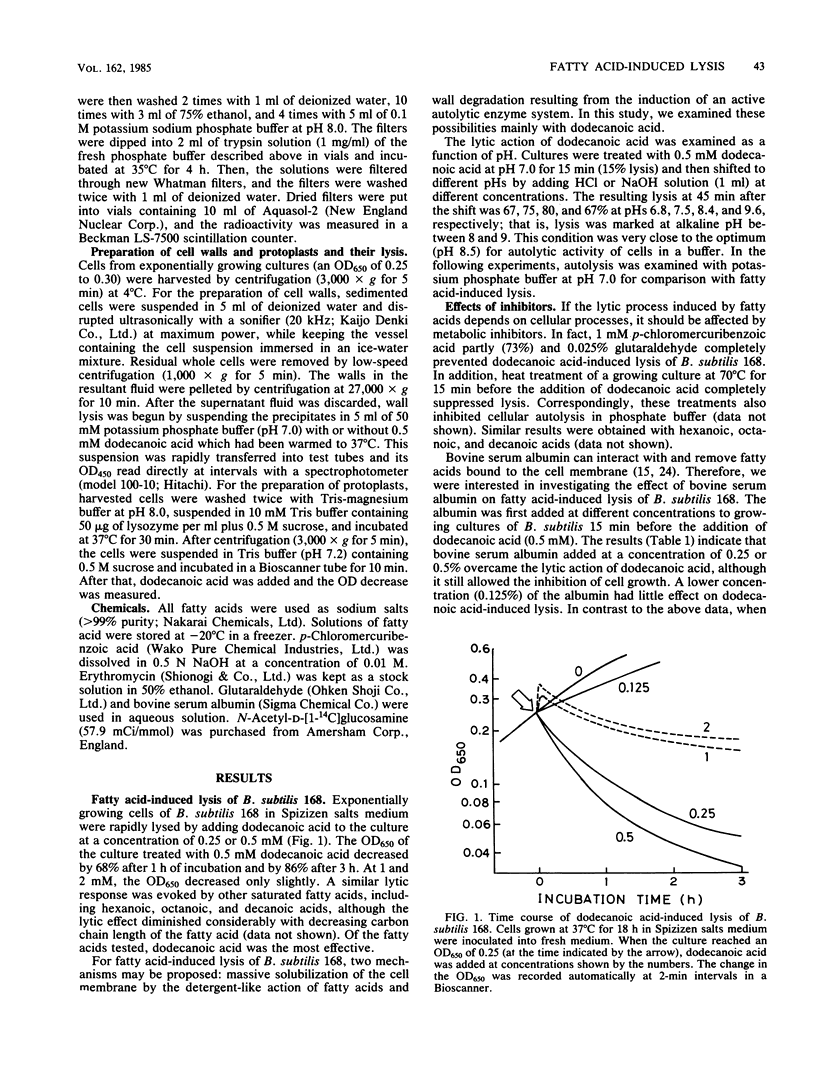
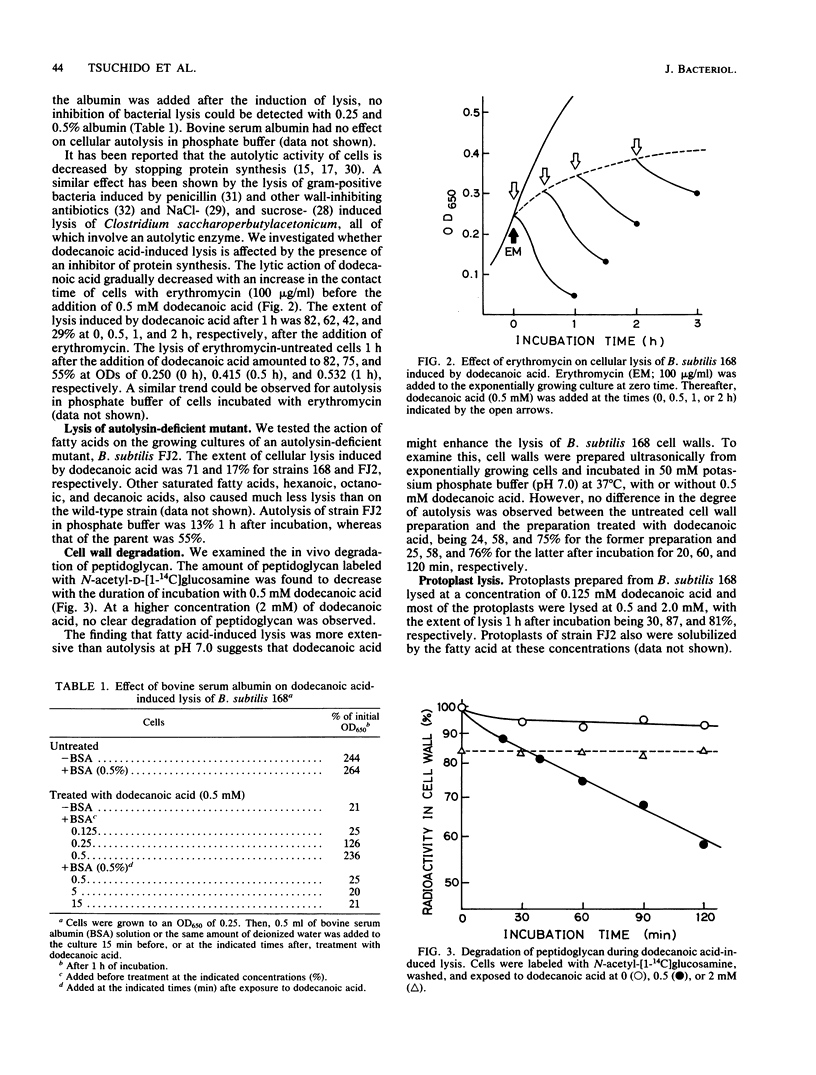
The addition of saturated C6, C8, C10, and C12 fatty acids appeared to lyse actively growing cells of Bacillus subtilis 168, as judged by a decrease in the optical density of the culture. Of these fatty acids, dodecanoic acid was the most effective, with 50% lysis occurring in about 30 min at a concentration of 0.5 mM. These conditions also decreased the amount of peptidoglycan estimated by the incorporated radioactivity of N-acetyl-D-[1-14C]glucosamine. At concentrations above 1 mM, however, bacterial lysis was not extensive. Dodecanoic acid did not affect autolysis of the cell wall. The lytic action of dodecanoic acid was greatly diminished in cells in which protein synthesis was inhibited and in an autolytic enzyme-deficient mutant. The results suggest that fatty acid-induced lysis of B. subtilis 168 is due to the induction of autolysis by an autolytic enzyme rather than massive solubilization of the cell membrane by the detergent-like action of the fatty acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson D. D., Daneo-Moore L. Effects of fatty acids on lysis of Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1122–1126. doi: 10.1128/jb.141.3.1122-1126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Shockman G. D. Cellular lysis of Streptococcus faecalis induced with triton X-100. J Bacteriol. 1978 Jul;135(1):153–160. doi: 10.1128/jb.135.1.153-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON I. M., LOMINSKI I., STERN H. An electron-microscope study of the action of cetyl-trimethyl-ammonium bromide on Staphylococcus aureus. J Pathol Bacteriol. 1953 Oct;66(2):513–526. doi: 10.1002/path.1700660223. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. New centrifugation technique for isolating enzymes from large cell structures: isolation and characterization of two Bacillus subtilis autolysins. J Bacteriol. 1972 Mar;109(3):1258–1265. doi: 10.1128/jb.109.3.1258-1265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay J. P., Farías R. N. Inhibitory action of a non-metabolizable fatty acid on the growth of Escherichia coli: role of metabolism and outer membrane integrity. J Bacteriol. 1977 Dec;132(3):790–795. doi: 10.1128/jb.132.3.790-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Ward J. B. N-acetylmuramyl-L-alanine amidase of Bacillus licheniformis and its L-form. J Bacteriol. 1972 Jun;110(3):878–888. doi: 10.1128/jb.110.3.878-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Freese E., Sheu C. W., Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973 Feb 2;241(5388):321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- Galbraith H., Miller T. B. Effect of long chain fatty acids on bacterial respiration and amino acid uptake. J Appl Bacteriol. 1973 Dec;36(4):659–675. doi: 10.1111/j.1365-2672.1973.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Galbraith H., Miller T. B. Effect of metal cations and pH on the antibacterial activity and uptake of long chain fatty acids. J Appl Bacteriol. 1973 Dec;36(4):635–646. doi: 10.1111/j.1365-2672.1973.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Galbraith H., Miller T. B. Physicochemical effects of long chain fatty acids on bacterial cells and their protoplasts. J Appl Bacteriol. 1973 Dec;36(4):647–658. doi: 10.1111/j.1365-2672.1973.tb04150.x. [DOI] [PubMed] [Google Scholar]
- Goto K., Mizushima S. Removal by bovine serum albumin of fatty acids from membrane vesicles and its effect on proline transport activity in Escherichia coli. J Biochem. 1978 Aug;84(2):251–258. doi: 10.1093/oxfordjournals.jbchem.a132125. [DOI] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975 May;122(2):385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Bacillus subtilis N-acetylmuramic acid L-alanine amidase. J Biol Chem. 1975 Mar 10;250(5):1676–1682. [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Site of initiation of cellular autolysis in Streptococcus faecalis as seen by electron microscopy. J Bacteriol. 1970 Aug;103(2):504–512. doi: 10.1128/jb.103.2.504-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Kabara J. J., Swieczkowski D. M., Conley A. J., Truant J. P. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972 Jul;2(1):23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E., Kanai K. Further studies on the lethal effect of long-chain fatty acids on mycobacteria. Jpn J Med Sci Biol. 1976 Feb;29(1):25–37. doi: 10.7883/yoken1952.29.25. [DOI] [PubMed] [Google Scholar]
- Levin B. C., Freese E. Comparison of the effects of two lipophilic acids, hexachlorophene and decanoate, on Bacillus subtilis. Antimicrob Agents Chemother. 1977 Sep;12(3):357–367. doi: 10.1128/aac.12.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- NIEMAN C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954 Jun;18(2):147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S., Hongo M. Lysis induced by sodium ion and its relation to lytic enzyme systems in Clostridium saccharoperbutylacetonicum. J Gen Microbiol. 1974 Apr;81(2):315–323. doi: 10.1099/00221287-81-2-315. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the location of autolysin, cell wall synthesis, and the development of resistance to cellular autolysis in Streptococcus faecalis after inhibition of protein synthesis. J Bacteriol. 1970 Aug;103(2):457–466. doi: 10.1128/jb.103.2.457-466.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Taylor C., Reeve J. N. Mucopeptide synthesis by rod mutants of Bacillus subtilis. J Gen Microbiol. 1974 Dec;85(2):335–349. doi: 10.1099/00221287-85-2-335. [DOI] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Effects of fatty acids on growth and envelope proteins of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):516–524. doi: 10.1128/jb.111.2.516-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Konings W. N., Freese E. Effects of acetate and other short-chain fatty acids on sugar and amino acid uptake of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):525–530. doi: 10.1128/jb.111.2.525-530.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Salomon D., Simmons J. L., Sreevalsan T., Freese E. Inhibitory effects of lipophilic acids and related compounds on bacteria and mammalian cells. Antimicrob Agents Chemother. 1975 Mar;7(3):349–363. doi: 10.1128/aac.7.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]


