Abstract
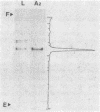
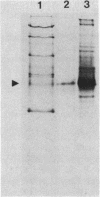
Component A2 of the methylcoenzyme M methylreductase system of Methanobacterium thermoautotrophicum has been purified 370-fold by liquid chromatography. Homogeneity was obtained by anaerobic preparative polyacrylamide gel electrophoresis. Component A2 is a colorless, air-stable protein consisting of a single polypeptide as indicated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The relative molecular mass of the native protein was determined by high-performance, size exclusion chromatography to be Mr 52,000; on sodium dodecyl sulfate-polyacrylamide gel electrophoresis a value of Mr 59,000 was obtained. When cell extract was subjected to N6-ATP-agarose affinity chromatography the methylcoenzyme M methylreductase system was resolved into two fractions; one of them was component A2. This work provides a new operational definition for component A2, i.e., its characteristic chromatographic behavior on N6-ATP-agarose. However, its functional definition is its ability to reconstitute the methylreductase activity with components A1, A3, and C. Several attempts to assign a role to component A2 are reported.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Role of component C in the methylreductase system of Methanobacterium. J Biol Chem. 1980 Sep 25;255(18):8388–8389. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Tandon S. M., Wolfe R. S. A procedure for anaerobic column chromatography employing an anaerobic Freter-type chamber. Anal Biochem. 1980 Jan 15;101(2):327–331. doi: 10.1016/0003-2697(80)90195-5. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Jacobson F. S., Daniels L., Fox J. A., Walsh C. T., Orme-Johnson W. H. Purification and properties of an 8-hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. J Biol Chem. 1982 Apr 10;257(7):3385–3388. [PubMed] [Google Scholar]
- KUNITZ M. Crystalline inorganic pyrophosphatase isolated from baker's yeast. J Gen Physiol. 1952 Jan;35(3):423–450. doi: 10.1085/jgp.35.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard L. H. Gradient sievorptive chromatography. A focusing system for the separation of cellular components. Biochemistry. 1973 Sep 11;12(19):3627–3632. doi: 10.1021/bi00743a009. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagle D. P., Jr, Wolfe R. S. Component A of the methyl coenzyme M methylreductase system of Methanobacterium: resolution into four components. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2151–2155. doi: 10.1073/pnas.80.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. M., Wolfe R. S. ATP requirement for methanogenesis in cell extracts of methanobacterium strain M.o.H. Biochim Biophys Acta. 1969 Dec 30;192(3):420–429. doi: 10.1016/0304-4165(69)90391-2. [DOI] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. A simplified assay for coenzyme M (HSCH2CH2SO3). Resolution of methylcobalamin-coenzyme M methyltransferase and use of sodium borohydride. J Biol Chem. 1974 Aug 10;249(15):4886–4890. [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Activation of the methylreductase system from Methanobacterium bryantii by ATP. J Bacteriol. 1983 May;154(2):640–649. doi: 10.1128/jb.154.2.640-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]