Abstract
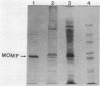
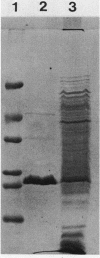
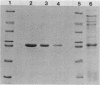
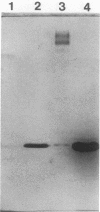
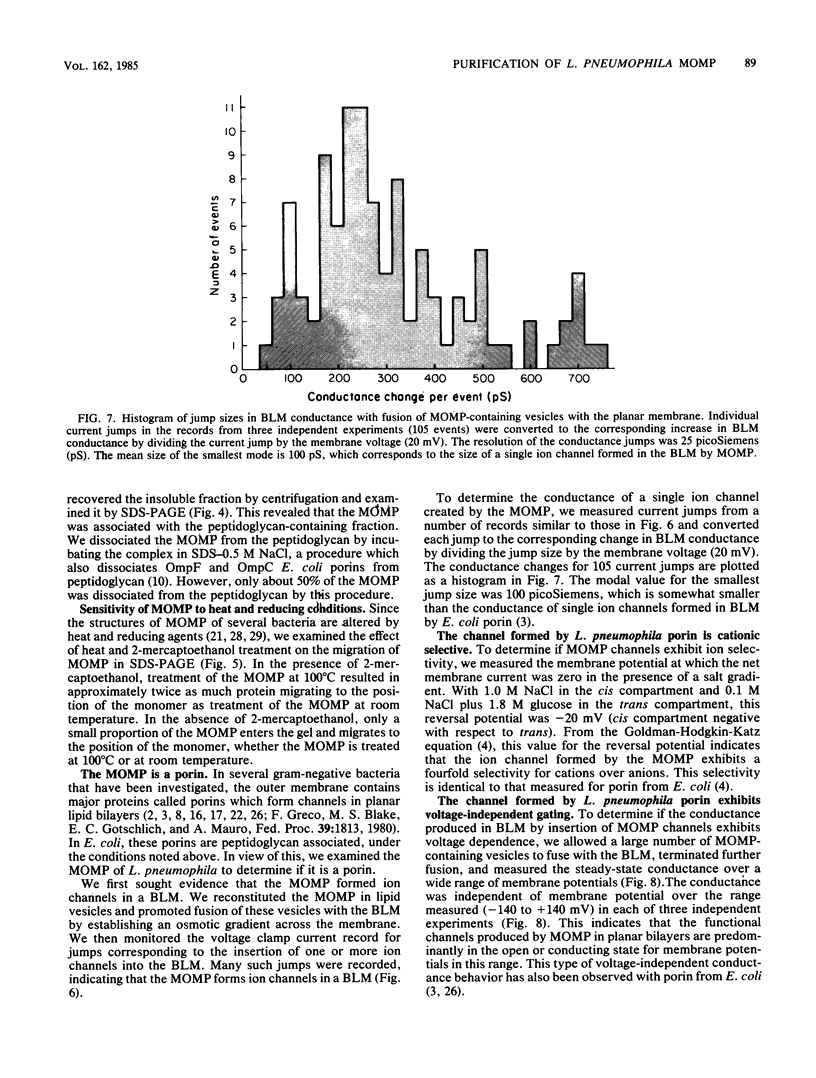
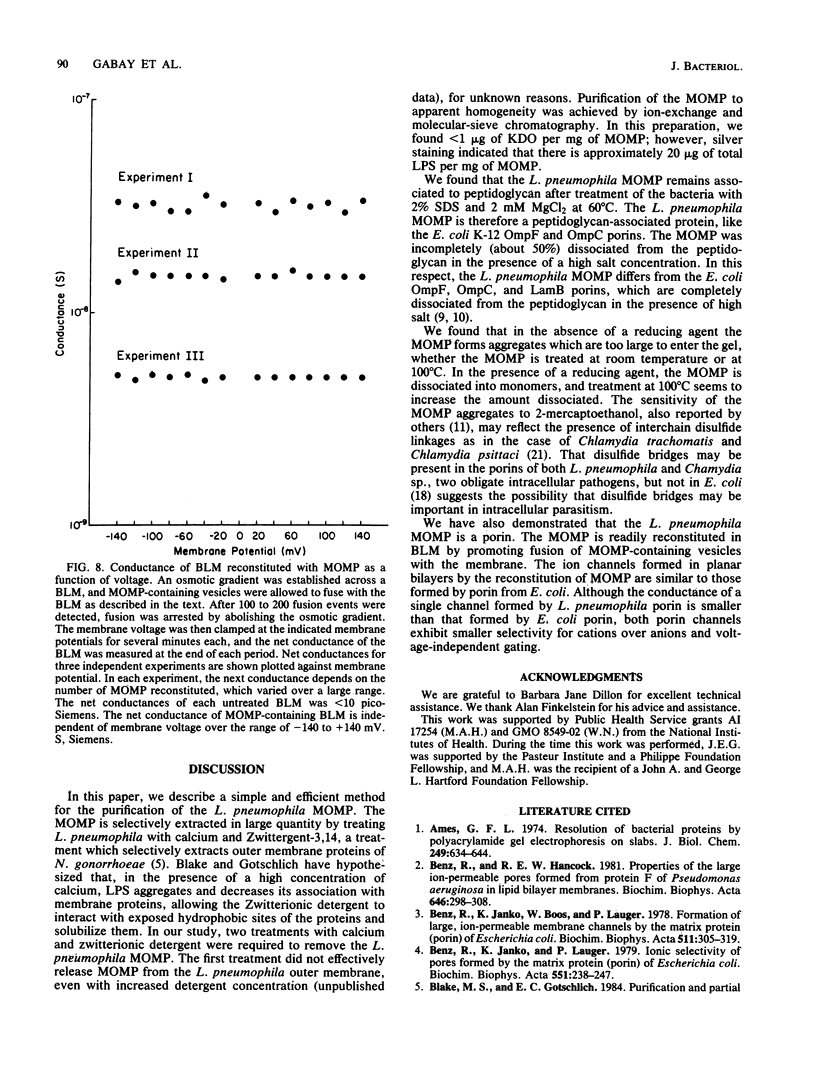
We have purified the major outer membrane protein (MOMP) of Legionella pneumophila, determined that it is associated with peptidoglycan, and characterized it as a porin. To purify the MOMP, we used a simple, rapid, three-step procedure that gave us the protein in high yield. The first step of the purification procedure involved selectively extracting the MOMP from whole bacterial cells with calcium and zwitterionic detergent. The second and third steps achieved purification by ion-exchange and molecular-sieve chromatography. The dissociation of the MOMP into monomers was dependent upon the presence of a reducing agent and was enhanced by treatment at 100 degrees C. To study the relationship of the MOMP to peptidoglycan, we extracted the protein by a modification of the Rosenbusch procedure. Like the Escherichia coli porins, the MOMP was peptidoglycan associated. The MOMP was at least partially dissociated from peptidoglycan in sodium dodecyl sulfate and a high salt concentration. To study the ion channel-forming properties of the MOMP, we reconstituted the MOMP in planar lipid membranes. The MOMP formed ion-permeable channels with a single-channel conductance size of 100 picoSiemens. The MOMP channels exhibited a fourfold selectivity for cations over anions and voltage-independent gating. These findings demonstrate that the MOMP is a porin with properties similar to those of E. coli porins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Läuger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1979 Mar 8;551(2):238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Henning U. Primary structure of major outer membrane protein I of Escherichia coli B/r. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5014–5017. doi: 10.1073/pnas.76.10.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Zimmerberg J., Finkelstein A. Parameters affecting the fusion of unilamellar phospholipid vesicles with planar bilayer membranes. J Cell Biol. 1984 Mar;98(3):1054–1062. doi: 10.1083/jcb.98.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Horwitz M. A. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila). J Exp Med. 1985 Feb 1;161(2):409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J., Yasunaka K. Interaction of the lamB protein with the peptidoglycan layer in Escherichia coli K12. Eur J Biochem. 1980 Feb;104(1):13–18. doi: 10.1111/j.1432-1033.1980.tb04393.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Hindahl M. S., Iglewski B. H. Isolation and characterization of the Legionella pneumophila outer membrane. J Bacteriol. 1984 Jul;159(1):107–113. doi: 10.1128/jb.159.1.107-113.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Intracellular multiplication of Legionnaires' disease bacteria (Legionella pneumophila) in human monocytes is reversibly inhibited by erythromycin and rifampin. J Clin Invest. 1983 Jan;71(1):15–26. doi: 10.1172/JCI110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Ishii J., Tokunaga M. Subunit structure of functional porin oligomers that form permeability channels in the other membrane of Escherichia coli. J Biol Chem. 1979 Mar 10;254(5):1457–1461. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nash T. W., Libby D. M., Horwitz M. A. Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Invest. 1984 Sep;74(3):771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J. M. The receptor protein of phage lambda: purification, characterization and preliminary electrical studies in planar lipid bilayers. Ann Microbiol (Paris) 1982 Jan;133A(1):27–32. [PubMed] [Google Scholar]
- Newhall W. J., Jones R. B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983 May;154(2):998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pine L., George J. R., Reeves M. W., Harrell W. K. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1979 May;9(5):615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]