Abstract
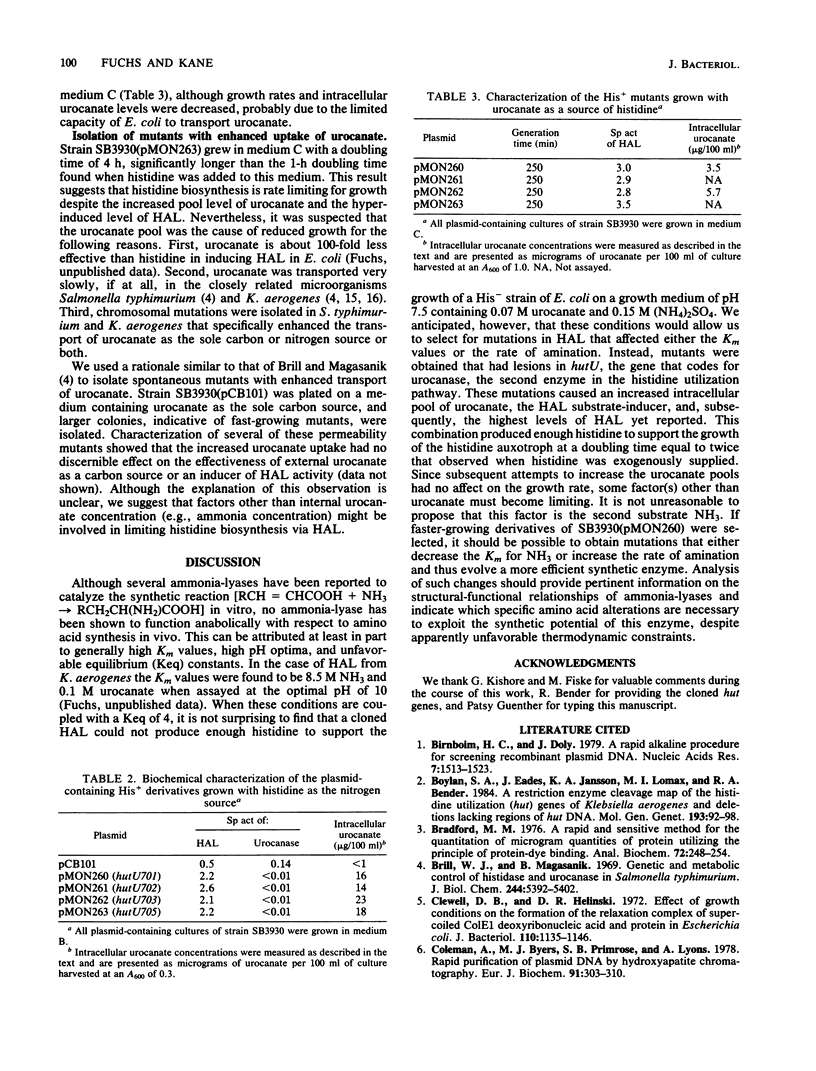
Histidine ammonia-lyase catalyzes the first step in histidine catabolism, the deamination of histidine to urocanate and ammonia. In vitro experiments have shown that histidine ammonia-lyase also can catalyze the reverse (amination) reaction, histidine synthesis, relatively efficiently under extreme reaction conditions (4 M NH4OH, pH 10). An Escherichia coli hisB deletion strain was transformed with a pBR322 derivative plasmid (pCB101) containing the entire Klebsiella aerogenes histidine utilization (hut) operon to determine whether the catabolic histidine ammonia-lyase could function biosynthetically in vivo to satisfy the histidine auxotrophy. Although the initial construct did not grow on media containing urocanate and ammonia as a source of histidine, spontaneous mutants possessing this ability were isolated. Four mutants characterized grew at doubling times of 4 h compared with 1 h when histidine was present, suggesting that histidine synthesis, although unequivocally present, remained growth limiting. Each mutant contained a plasmid-encoded mutation which eliminated urocanase activity, the second enzyme in the Hut catabolic pathway. This genetic block led to the accumulation of high intracellular levels of urocanate, which was subsequently converted to histidine via histidine ammonia-lyase, thus satisfying the histidine auxotrophic requirement.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan S. A., Eades L. J., Janssen K. A., Lomax M. I., Bender R. A. A restriction enzyme cleavage map of the histidine utilization (hut) genes of Klebsiella aerogenes and deletions lacking regions of hut DNA. Mol Gen Genet. 1984;193(1):92–98. doi: 10.1007/BF00327420. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brill W. J., Magasanik B. Genetic and metabolic control of histidase and urocanase in Salmonella typhimurium, strain 15-59. J Biol Chem. 1969 Oct 10;244(19):5392–5402. [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Byers M. J., Primrose S. B., Lyons A. Rapid purification of plasmid DNAs by hydroxyapatite chromatography. Eur J Biochem. 1978 Nov 2;91(1):303–310. doi: 10.1111/j.1432-1033.1978.tb20966.x. [DOI] [PubMed] [Google Scholar]
- Deshpande K. L., Katze J. R., Kane J. F. Effect of glutamine on enzymes of nitrogen metabolism in Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):768–774. doi: 10.1128/jb.145.2.768-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Hartman P. E. Histidine-requiring mutants of Escherichia coli K12. Genetics. 1970 Oct;66(2):231–244. doi: 10.1093/genetics/66.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Lund P., Neidhardt F. C., Schwartz D. T. Induction and repression of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4320–4324. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J Biol Chem. 1969 Oct 10;244(19):5382–5391. [PubMed] [Google Scholar]
- Schlesinger S., Magasanik B. Imidazolepropionate, a nonmetabolizable inducer for the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4325–4330. [PubMed] [Google Scholar]
- Schlesinger S., Scotto P., Magasanik B. Exogenous and endogenous induction of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4331–4337. [PubMed] [Google Scholar]
- Williams V. R., Hiroms J. M. Reversibility of the "irreversible" histidine ammonia-lyase reaction. Biochim Biophys Acta. 1967 May 16;139(1):214–216. doi: 10.1016/0005-2744(67)90139-8. [DOI] [PubMed] [Google Scholar]
- Yamada S., Nabe K., Izuo N., Nakamichi K., Chibata I. Production of l-Phenylalanine from trans-Cinnamic Acid with Rhodotorula glutinis Containing l-Phenylalanine Ammonia-Lyase Activity. Appl Environ Microbiol. 1981 Nov;42(5):773–778. doi: 10.1128/aem.42.5.773-778.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


