Abstract
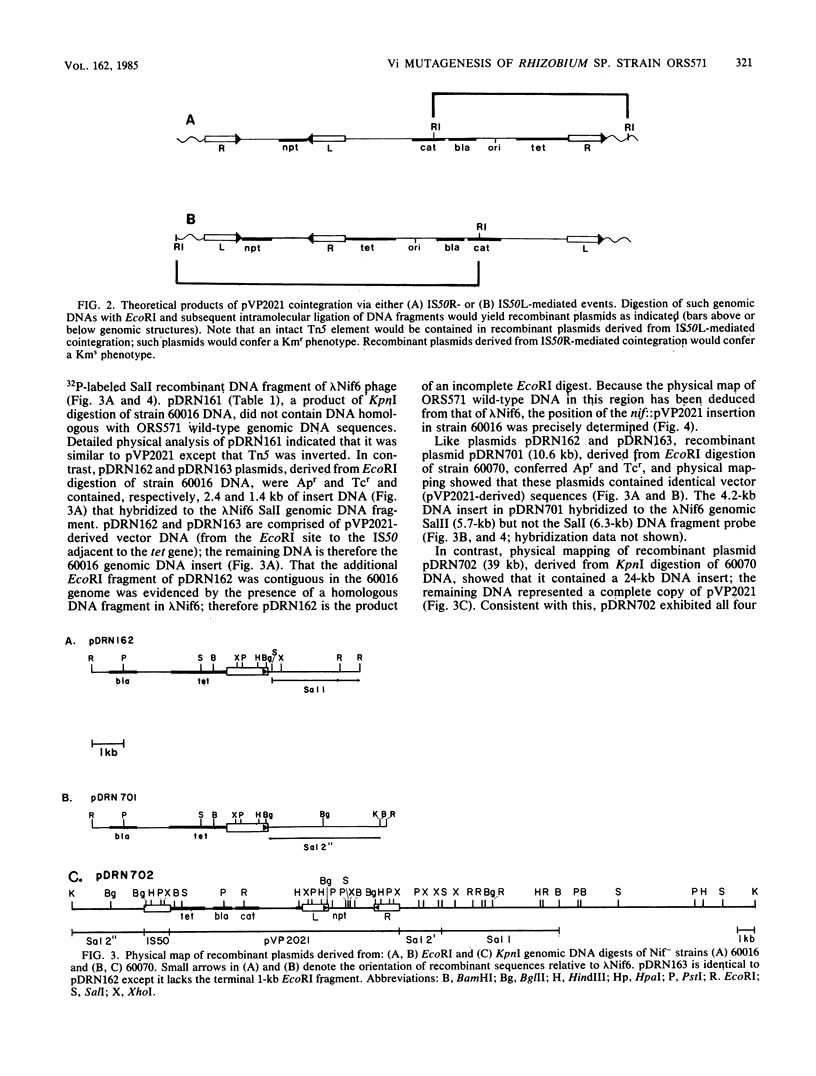
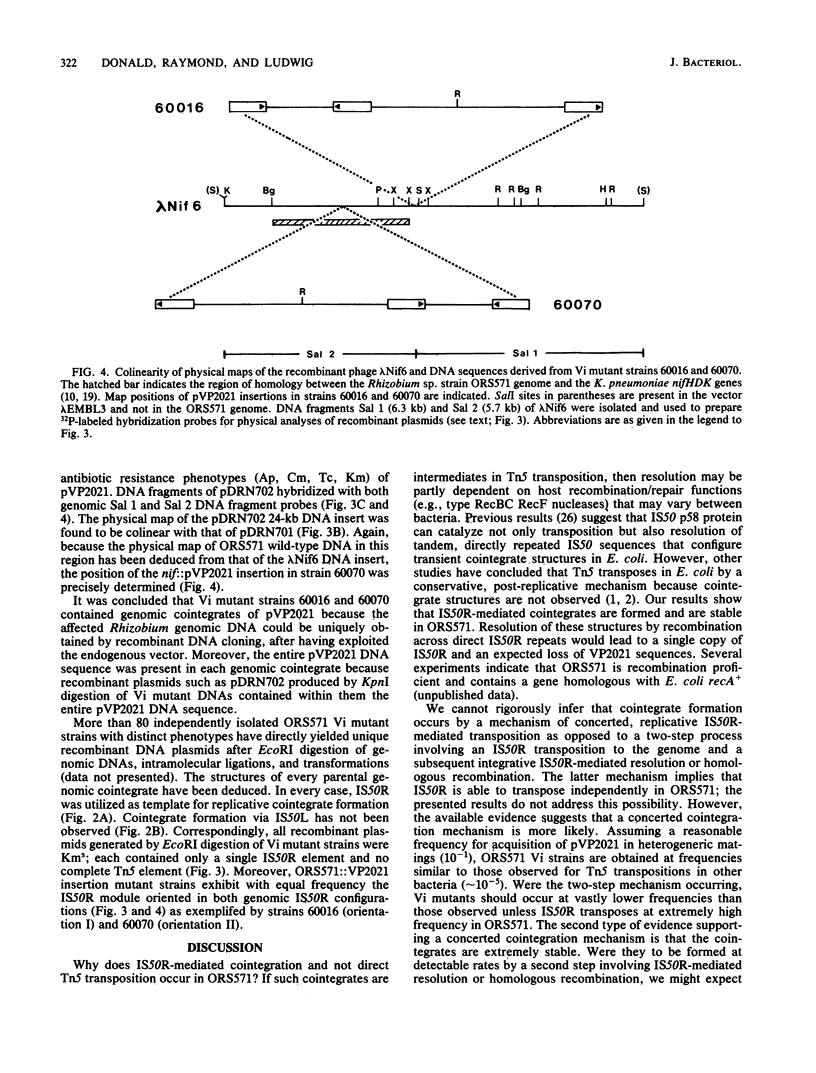
When the limited-host-range plasmid pVP2021 carrying Tn5 was mobilized into Rhizobium sp. strain ORS571 and stable acquisition of Tn5 was selected, ORS571 plasmid-genome cointegrates were exclusively obtained; direct Tn5 transposition was never observed. In every case, genomic cointegrates exhibited an additional (third) IS50 element that bordered VP2021 DNA sequences but maintained a single Tn5 element. Genomic cointegrates containing IS50 triplications were stable; neither phenotypic reversion nor resolution was detectable. Auxotrophic mutant strains (vector insertion mutants) were identified at expected frequencies among derivatives carrying ostensibly random genomic pVP2021 insertions; N2 fixation (Nif)-defective vector insertion mutants were observed among these derivatives at a frequency of 10(-3). The presence of integrated pVP2021 in ORS571 nif::VP2021 mutant genomes enabled VP2021 to constitute an endogenous cloning vector. After EcoRI or KpnI digestions, genomic nif::pVP2021 DNA sequences contiguous with integrated pVP2021 were directly cloned as new replicons without addition of an exogenous vector. Recombinant plasmids derived from two such nif::pVP2021 mutants hybridized to previously analyzed ORS571 Nif DNA sequences. Recombinant plasmid DNA and ORS571 Nif region DNA were found to be colinear; pVP2021 insertions could be accurately mapped. pVP2021 insertion-mutagenesis thus allows the direct cloning of ORS571 gene sequences for which mutant phenotypes can be selected or screened.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Johnsrud L., McDivitt L., Ramabhadran R., Hirschel B. J. Inverted repeats of Tn5 are transposable elements. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2632–2635. doi: 10.1073/pnas.79.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E. Structural requirement for IS50-mediated gene transposition. Proc Natl Acad Sci U S A. 1983 Feb;80(3):792–796. doi: 10.1073/pnas.80.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R. G., Ludwig R. A. Rhizobium sp. strain ORS571 ammonium assimilation and nitrogen fixation. J Bacteriol. 1984 Jun;158(3):1144–1151. doi: 10.1128/jb.158.3.1144-1151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Elmerich C., Dreyfus B. L., Reysset G., Aubert J. P. Genetic analysis of nitrogen fixation in a tropical fast-growing Rhizobium. EMBO J. 1982;1(4):499–503. doi: 10.1002/j.1460-2075.1982.tb01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979 Jan;2(1):1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Lazaar A. L., Syvanen M. Regulation of Tn5 by the right-repeat proteins: control at the level of the transposition reaction? Cell. 1982 Oct;30(3):883–892. doi: 10.1016/0092-8674(82)90293-8. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Yin J. C., Reznikoff W. S. Control of Tn5 transposition in Escherichia coli is mediated by protein from the right repeat. Cell. 1982 Oct;30(3):873–882. doi: 10.1016/0092-8674(82)90292-6. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnoky P., Kiss G. B., Ott I., Kondorosi A. Tn5 carries a streptomycin resistance determinant downstream from the kanamycin resistance gene. Mol Gen Genet. 1983;191(2):288–294. doi: 10.1007/BF00334828. [DOI] [PubMed] [Google Scholar]
- Riedel G. E., Ausubel F. M., Cannon F. C. Physical map of chromosomal nitrogen fixation (nif) genes of Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2866–2870. doi: 10.1073/pnas.76.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess G., Masepohl B., Puehler A. Analysis of IS21-mediated mobilization of plasmid pACYC184 by R68.45 in Escherichia coli. Plasmid. 1983 Sep;10(2):111–118. doi: 10.1016/0147-619x(83)90063-x. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Reznikoff W. S. The functional differences in the inverted repeats of Tn5 are caused by a single base pair nonhomology. Cell. 1981 Jan;23(1):191–199. doi: 10.1016/0092-8674(81)90284-1. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Carle G. F., Berg D. E. Sequences essential for transposition at the termini of IS50. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7293–7297. doi: 10.1073/pnas.80.23.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. T., Finnerty W. R. Insertional specificity of transposon Tn5 in Acinetobacter sp. J Bacteriol. 1984 Feb;157(2):607–611. doi: 10.1128/jb.157.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic T. J., Marvo S. L., Chung J. H., Peralta E. G., Jaskunas S. R. RecA-independent recombination between direct repeats of IS50. Cell. 1983 Jun;33(2):629–637. doi: 10.1016/0092-8674(83)90444-0. [DOI] [PubMed] [Google Scholar]
- de Vries G. E., Raymond C. K., Ludwig R. A. Extension of bacteriophage lambda host range: selection, cloning, and characterization of a constitutive lambda receptor gene. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6080–6084. doi: 10.1073/pnas.81.19.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]


