Abstract
Background and purpose:
Epithelial injury contributes to lung pathogenesis. Our work and that of others have identified the phosphoinositide-3 kinase (PI3K)/Akt pathway as a vital component of survival in lung epithelia. Therefore, we hypothesized that pharmacological inhibition of PTEN, a major suppressor of this pathway, would enhance wound closure and restore lung epithelial monolayer integrity following injury.
Experimental approach:
We evaluated the ability of two bisperoxovanadium derivatives, bpV(phen) and bpV(pic), in differentiated primary human airway epithelia and BEAS2B cultures for their ability to inhibit PTEN, activate the PI3K/Akt pathway and restore epithelial monolayer integrity following mechanical injury.
Key results:
BpV(phen) and bpV(pic) induced Akt phosphorylation in primary and BEAS2B cells in a dose and time dependent manner. Minimal toxicity was observed as measured by lactate dehydrogenase (LDH) release. To verify that Akt phosphorylation is specifically induced by PTEN inhibition, the PTEN positive cell line, DU145, and two PTEN negative cell lines, LNCaP and PC3, were examined. PTEN positive cells demonstrated a dose responsive increase in Akt phosphorylation whereas PTEN negative cells showed no response indicating that bpV(phen) directly suppresses PTEN without affecting auxiliary pathways. Next, we observed that exposure to either compound resulted in accelerated wound closure following mechanical injury. Similar effects were observed after transfection with a dominant negative isoform of PTEN and PTEN specific siRNA.
Conclusions and implications:
From these studies, we conclude that PTEN is a valid target for future studies directed at restoring epithelial barrier function after lung injury.
Keywords: PTEN, lung, epithelium, wound remodelling
Introduction
The airway epithelium forms an essential barrier that maintains tissue homoeostasis, prevents entry by foreign bodies and facilitates optimal gas exchange (Mutlu and Sznajder, 2005; Jain and Eaton, 2006). Repair of the airway epithelium following injury is critical for the maintenance of barrier function and to limit airway hyper-reactivity in response to external stimuli. Defective wound repair in susceptible individuals is a major contributing factor to commonly occurring lung diseases, including asthma and chronic obstructive pulmonary disease, yet no therapeutic agents are available to directly stimulate tissue repair (Davies et al., 2003; Puchelle et al., 2006). Therefore, the development of novel therapeutic strategies that promote epithelial wound repair and regeneration is warranted.
The phosphoinositide-3 kinase (PI3K)/Akt signal transduction pathway is a potent inducer of cell survival (Ray et al., 2003). Akt is a well-characterized mediator of many cellular processes including growth, survival, protein synthesis, cell cycle regulation and apoptosis (Chan et al., 1999). We previously reported that Akt activation protects the lung epithelium from receptor-mediated apoptosis (Bao et al., 2005). A related study reported that overexpression of a constitutively active form of Akt in the lung protects mice from hyperoxic pulmonary damage and mortality (Lu et al., 2001). In addition, a series of investigations have shown that Akt activation by keratinocyte growth factor plays a critical role in lung epithelial cell protection in vitro and in vivo under conditions of oxidative stress (Ray et al., 2003; Ray, 2005). In view of these observations, we proposed that investigation of this signalling pathway would provide a unique opportunity to protect or restore the lung epithelium, thereby creating a potentially innovative strategy to treat acute lung injury.
The protein PTEN (phosphatase and tensin homologue deleted on chromosome ten) is a counter-regulator of the PI3K/Akt pathway and acts by dephosphorylation of phosphatidyl-inositol 3,4,5-trisphosphate (PI(3,4,5)P3) at the 3′-position of the inositol ring, thereby inactivating the second messenger of this survival pathway (Maehama and Dixon, 1998). This is relevant in the context of human disease since it is known that PTEN deficiency, by either polymorphic mutation or gene deletion, is a cause of cancer (Stambolic et al., 1998; Tamura et al., 1999). Although loss of PTEN function is associated with poor outcomes, temporary phosphatase suppression may be beneficial. We and others have shown that activation of PI3K/Akt signalling promotes lung epithelial cell survival (Bao et al., 2005) and we have recently observed that PTEN is constitutively abundant in the lung epithelium. These observations suggest that PTEN is a chronic suppressor of Akt-mediated signalling in the lung epithelia, normally a fully differentiated, non-dividing cell type. In view of this, we hypothesized that temporary suppression of PTEN, through pharmacological manipulation, would activate Akt-mediated signalling events culminating in enhanced lung epithelial wound repair.
Vanadium was first recognized as a competitive, reversible, inhibitor of tyrosine phosphatases (Gordon, 1991; Huyer et al., 1997). New and improved analogues such as the bisperoxovanadium compounds have since been developed through derivatization of the vanadium parent compound. These compounds pharmacologically interact with PTEN by binding to the CX5R motif, a reactive centre shared by other protein tyrosine phosphatases. However, in comparison to vanadium, the bisperoxovanadium derivatives are more specific for PTEN. As a proof of this, a recent study reported a distinct IC50 for PTEN approximately 10- to100-fold lower, in comparison to other tyrosine phosphatases, that stimulated Akt phosphorylation (Schmid et al., 2004). In addition, potassium bisperoxo (1,10-phenanthroline) oxovanadate (bpV(phen)) has been shown to induce cell survival through activation of the mitogen-activated protein kinase system, whereas cell death occurred at higher concentrations due to activation of the JNK-c-jun cascade, thereby demonstrating dose-dependent dual effects (Cerovac et al., 1999; Rumora et al., 2004).
In view of these observations, the bisperoxovanadium compounds, as a class, merit further investigation as potent inhibitors of PTEN. Due to their specificity towards PTEN, we hypothesized that these agents have potential as therapeutic agents to enhance lung epithelial wound repair. As a first step, we have evaluated two vanadate derivatives, bpV(phen) and di-potassium bisperoxo (picolinato) oxovanadate (bpV(pic)), in primary human and epithelial cell line cultures. Initially, we observed that both the compounds exhibited dose- and time-dependent induction of Akt phosphorylation at relatively low, physiologically attainable, doses without apparent toxicity. Furthermore, at these lower doses, the compounds were specific for the intended drug target and most importantly, enhanced epithelial wound closure. Additional molecular studies were conducted to further validate PTEN as a viable drug target in the lung epithelium. In view of our findings, we contend that additional investigation involving PTEN inhibition by bisperoxovanadium compounds is warranted and may provide an innovative therapeutic strategy to promote lung epithelial cell repair following injury.
Methods
Cell cultures
Primary human upper airway epithelial cells (hUAECs), a transformed lung epithelial cell line (BEAS2B), a PTEN functional prostate cancer cell line (DU145) and two PTEN-null cell lines (LNCaP and PC3) were used in this study. Human lungs were collected with approval from The Ohio State University Institutional Review Board. Results in this investigation were derived from samples from two different donors. hUAECs were isolated following enzymatic dissociation from the trachea, bronchi and bronchioles of adult donor lungs, seeded onto collagen-coated, semi-permeable membranes (0.6 cm2; Millicell-HA, Millipore, Bedford, MA, USA) and grown at an air–liquid interface as previously described (Karp et al., 2002).
In all experiments, primary cultures were evaluated at a minimum of 2 weeks after initial seeding. hUAECs were maintained in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium, supplemented with 2% Ultroser G (BioSepra; Villeneuve, La Garenne, France) and antibiotics, unless otherwise stated. For the scrape wound model, primary cells were seeded onto 24-well plates in regular medium as described above and allowed to attach and form a monolayer before applying the scrape wound. BEAS2B cells (American Type Culture Collection) were cultured in small airway growth medium with 2% fetal bovine serum. DU145, LNCaP and PC3 cells were obtained from Dr Dalton's laboratory and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum. All cells were maintained at 37 °C in a humidified incubator with 5% carbon dioxide. Serum was routinely withdrawn from the cultures the day before each experiment to minimize growth factor-related signal activation and background noise.
Western blot analysis
Cell lysis buffer (Cell Signaling, Beverly, MA, USA) was directly added to cell cultures after removing medium and washing with cold phosphate-buffered solution (PBS) at designated time points. Whole-cell lysates were collected and centrifuged at 17 422 g for 10 min at 4 °C. Supernatant was measured for protein concentration (Bio-Rad, Hercules, CA, USA) and then mixed with loading buffer (Bio-Rad). Samples were boiled at 100 °C for 5 min, separated on 12% polyacrylamide gel electrophoresis gel (Bio-Rad) and transferred onto nitrocellulose membranes (Amersham Bioscience, Buckinghamshire, UK). Membranes were blocked with 5% (w/v) non-fat milk (Bio-Rad Laboratories) in Tris-buffered saline/0.1% Tween-20 for 1 h at room temperature followed by incubation with primary antibodies overnight at 4 °C. After washing, membranes were incubated with secondary antibody for 1 h at room temperature and developed (ECL kit; Amersham Bioscience). Blots were analysed quantitatively by standard densitometry. The antibodies utilized in this study were anti-Akt (1:2000; Cell Signaling); anti-p-Akt-Ser473 (1:1000; Cell Signaling); anti-PTEN (1:1000; Cell Signaling); anti-β-actin (1:5000; MP Biomedicals, Solon, OH, USA); anti-glycogen synthase kinase-3 (GSK3; 1:1000; Cell Signaling); anti-p-GSK3 (1:1000; Cell Signaling); goat anti-mouse immunoglobulin G-HRP (1:3000; Cell Signaling) and goat anti-rabbit immunoglobulin G-HRP (1:3000; Zymed, San Francisco, CA, USA). The ratio of phosphorylated Akt to total Akt (pAkt/Akt) was evaluated to normalize the total protein levels for each treatment group and to obtain the relative change in Akt.
Measurement of cell viability
Two conventional methods, a cell proliferation assay (water-soluble tetrazolium salt; WST-1) and a cell toxicity assay (lactate dehydrogenase (LDH)), were utilized to determine cell viability. As an indicator of cell proliferation or viability, WST-1 is cleaved by mitochondrial dehydrogenases and produces a colour change that correlates with the number of viable cells. WST-1 reagent was added into cell culture and monitored as per the manufacturer's specifications. LDH release was also determined in compliance with the manufacturer's instructions. Triton (2%) was used to lyse cells prior to LDH measurement and served as the positive control (100% LDH release).
Glutathione assay
Glutathione is an indicator of protein sulphhydryl reduction status so depletion of glutathione was used as an indirect measure to determine compound specificity. Since sulphhydryl groups reside in the functional site of PTEN, tyrosine phosphatases, and other phosphatases, a high level of depletion would indicate a lack of specificity with the peroxovanadium compounds. However, since PTEN represents a minor fraction of all phosphatases, significant changes in glutathione levels would not be expected if the compounds were specific for PTEN. Cells were cultured under regular conditions then lysed at the designated time points after the addition of compounds. Glutathione concentrations were determined following the manufacturer's instructions.
Scrape wound model under submersion conditions
The simian vacuolating virus 40 (SV40)-transformed, non-cancerous human bronchial epithelial cell line, BEAS2B, and primary cell cultures were used for this study. Initially, 0.2 × 106 BEAS2B cells per well or 1 × 106 hUAECs cells per well were seeded onto 24-well plates and allowed to establish monolayers, then replaced with serum-free medium the day before each experiment. A reproducible mechanical scrape wound was made in fully confluent monolayers with a 600-μm width pipette tip. Fresh medium, with or without PTEN inhibitors, was replaced immediately following removal of floating cells and debris after the scrape wound was made. The original width of the scrape wound was immediately measured for comparison with later time points. The width of the scrape wound (600 μm) allowed a complete view that contained both margins, thereby enabling the measurement of the entire wound over time. Live cell images were taken at designated time points between 0 and 24 h using automated time-lapse microscopy. During the observation period, cell cultures were maintained at 37 °C and 5% CO2 using a special chamber positioned on the microscope stage (Solent Scientific, Segensworth, UK). Measurements of the scrape width were randomly made across the lesion at a minimum of three points. Wound closure rate (growth distance per unit time) was analysed and compared among different treatments. Images were obtained using an Olympus BX61 Disc-scanning confocal microscope equipped with a Hamamatsu charge-coupled device camera and SlideBook 2D/3D time-lapse imaging software.
Migration assay
Migration chambers with an 8 μm pore size, commonly utilized for epithelial cell migration studies, were used in our assay (Millipore, Billerica, MA, USA). Inserts were first placed into a 24-well plate and hydrated with PBS on both the apical and basolateral surface before use. PBS was removed immediately prior to plating cells. Then 0.25 × 106 BEAS2B cells were suspended in 200 μl Dulbecco's modified Eagle's medium with or without drug treatment and then plated onto the apical surface of the inserts with 400 μl Dulbecco's modified Eagle's medium added to the basolateral chamber. Cultures were then incubated at 37 °C for up to 4 h to allow migration. At designated time points, the medium on both sides was removed and the inserts were submerged in 400 μl 0.25% trypsin-EDTA (Invitrogen Corporation, Carlsbad, CA, USA) and incubated for 5 min to dissociate migrating cells that were attached to the basolateral surface. Inserts were then removed and discarded. Regular medium (400 μl) containing 10% serum was placed in each well followed by centrifugation of the supernatants at 800 g for 5 min. Cell pellets were then resuspended into 50 μl PBS and counted.
Mechanical wound model for differentiated hUAECs
Differentiated hUAECs were cultured at an air-to-liquid interface. A mechanical, concentric scrape wound was reproducibly achieved using a pipette tip. The transwell surface was washed with PBS three times to remove all cell debris immediately after the wound was generated. Trans-epithelial electrical resistance (TEER) was evaluated to determine monolayer integrity out to 6 days after establishing the wound. A nonlinear regression curve fit was utilized using GraphPad Prism 5.0 software to determine TEER recovery. Evaluation of the TEER plateau followed by one-phase association under exponential function was utilized for kinetic analysis. Metric analysis of TEER recovery is expressed as K, the exponential rate constant for TEER recovery; T50, the time to 50% recovery and as the slope1−2d, the slope of the linear regression line between days 1 and 2. These values were compared among different treatment groups.
Modulation of PTEN expression
Transient transfection with a dominant negative (DN) PTEN plasmid and siRNA-mediated suppression of PTEN expression were also conducted in BEAS2B cells to evaluate further the role of PTEN in epithelial wound closure. For studies involving plasmid DNA transfection, a plasmid encoding the PTEN C124S mutant (Wu et al., 2000), which produces a phosphatase-dead PTEN, including both lipid and protein phosphatase activity, was utilized (Weng et al., 2001). Empty pcDNA3 vector (Invitrogen Corporation) transfection served as a negative control. In addition, an enhanced green fluorescence protein plasmid (Clontech Laboratories Inc., Mountain View, CA, USA) was co-transfected with the mutant to determine transfection efficiency, which typically exceeded 70%. Initially, 0.1 × 106 cells were seeded onto each well on a 24-well plate with regular medium 1 day prior to transfection. Cells were then rinsed with fresh Dulbecco's modified Eagle's medium before transfection as per the manufacturer's recommendation (Invitrogen Corporation). For siRNA studies, we first utilized a non-silencing, fluorescent-tagged oligomer to optimize delivery efficiency (Qiagen, Valencia, CA, USA). The same oligomer, which has no known homology to mammalian genes, was used as a negative control in subsequent experiments. A PTEN-specific siRNA (Qiagen) was then used under optimal transfection conditions to suppress endogenous PTEN expression. Transfection (delivery) efficiency was evaluated at 24 h, whereas optimal PTEN suppression was observed to occur at 48 h following siRNA transfection. To accomplish this, cells were lysed and analysed by western blotting to determine the extent of PTEN inhibition. Here, 70% or greater suppression was routinely achieved.
Statistical analysis
Data are given as means±s.d. The Student's two-sample t-test was used to compare the treatment groups with relevant controls. Analysis of variance was used to evaluate dose–response and time-course studies as well as other experiments involving more than two study groups. P-values <0.05 were taken to show significant differences between means.
Materials
The bisperoxovanadium derivatives, bpV(phen) and bpV(pic), were purchased from Alexis Biochemicals (San Diego, CA, USA). Sodium orthovanadate (Na3VO4) was purchased from Sigma Chemical Co. (Louis, MO, USA) and 30% hydrogen peroxide (H2O2) was purchased from Mallinckrodt Baker Inc. (Paris, KY, USA). A 1:1 dilution of Na3VO4 and 30% H2O2 was mixed and incubated at 37 °C for 15 min to activate Na3VO4 and served as a positive control for the glutathione assay. The cell proliferation assay (WST-1) and cell toxicity assay (LDH) kits were purchased from Roche Applied Sciences (Indianapolis, IN, USA) and used as per the manufacturer's guidelines. Triton X-100 (Sigma Chemical Co.) was used to lyse cells in the LDH kit. The glutathione assay was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Lipofectamine 2000 (Invitrogen Corporation) was used for transient transfection studies and Hyperfect (Qiagen) was used for siRNA-related studies.
Results
Activation of the PI3K/Akt pathway by PTEN inhibitors
Endogenous PTEN levels were first analysed in all cells. High PTEN protein levels were observed in both primary upper airway cultures and BEAS2B cells, and the PTEN-expressing cell line, DU145, while the PTEN-null cell line, LNCaP, showed no endogenous PTEN by western blotting (Figure 1a). These findings served as the basis for subsequent studies involving inhibition of PTEN. Since Akt is an immediate downstream target of PI3K and directly regulated by PTEN, it was used as a biomarker for PTEN activity. We first examined both primary upper airway cultures and BEAS2B cells for Akt phosphorylation following treatment with the PTEN inhibitor, bpV(phen). A linear dose–response occurred within 30 min of bpV(phen) treatment in both primary (Figure 1b) and BEAS2B cells (Figure 1c). We then compared the relative potency of both derivatives, bpV(phen) and bpV(pic), in the same culture conditions and observed similar findings, as reported previously, demonstrating that bpV(pic) is more potent than bpV(phen) in terms of Akt phosphorylation (Schmid et al., 2004) (Figure 1d). These results indicated that both PTEN inhibitors increase Akt phosphorylation in the lung epithelium at relatively low micromolar concentrations and within range of the previously reported IC50 for PTEN.
Figure 1.
Bisperoxovanadium compounds induced Akt phosphorylation. (a) PTEN (phosphatase and tensin homologue deleted on chromosome ten) was constitutively expressed in the lung epithelium. Endogenous PTEN, Akt and phosphorylated Akt protein levels were determined by western analysis in primary human upper airway epithelial cells (hUAECs) and BEAS2B cells, and compared to the PTEN functional cell line, DU145, and a PTEN-null cell line, LNCaP. (b) Potassium bisperoxo (1,10-phenanthroline) oxovanadate (bpV(phen)) induced a dose-dependent increase in Akt phosphorylation in hUAECs within 30 min. Quantitative analysis by densitometry is shown in the bottom panel following comparison of the ratio of phosphorylated Akt to total Akt. PC is the positive control. (c) Similar results are shown in BEAS2B cells following stimulation with bpV(phen) (0.1–10 μM). (d) Direct comparison of bpV(phen) and di-potassium bisperoxo (picolinato) oxovanadate in BEAS2B cells demonstrates that both compounds induced Akt phosphorylation at submicromolar concentrations. The western blots are representative of four experiments; other data are mean±s.d.; n=4.
Evaluation of cytotoxicity with PTEN inhibitors
Next, cell viability (WST-1) and cell toxicity (LDH) assays were utilized to evaluate the toxicity of both PTEN inhibitors. In general, minimal evidence of detrimental effects were observed within a dosage range of 0.5–5 μM for both PTEN inhibitors following 24-h treatment. bpV(phen) at the highest dose (5 μM) did exhibit minimal cytotoxicity as shown by a 1.4-fold increase in LDH release (Figure 2a). We also evaluated cell viability following prolonged exposure to both compounds. Again, bpV(pic) demonstrated no evidence of cytotoxicity for up to 48-h exposure while prolonged exposure to bpV(phen) at 2 or 5 μM resulted in a nominal increase in toxicity as measured by both LDH release and the WST-1 assay (Figure 2b). Furthermore, no detectable toxicity was observed in primary cultures under submersed culture conditions at a dose of 1–2 μM for up to 96 h after exposure to both compounds as measured by LDH release (Figure 2c). Under all conditions, we observed an increase in Akt phosphorylation that was maintained over 24 h demonstrating that each compound provided a sustained effect on the intended target without significant cell toxicity (Figure 2d).
Figure 2.
Prolonged exposure to bisperoxovanadium compounds is associated with minimal cytotoxicity. Cytotoxicity and cell viability were simultaneously determined by lactate dehydrogenase (LDH) and water-soluble tetrazolium salt assays, respectively, following prolonged exposure to potassium bisperoxo (1,10-phenanthroline) oxovanadate (bpV(phen)) and di-potassium bisperoxo (picolinato) oxovanadate (bpV(pic)). In view of the previous results, we evaluated a dosage range between 1 and 5 μM for bpV(phen) and 0.5–5 μM for bpV(pic). (a) Minimal cytotoxicity or alteration in viability was observed following 24-h exposure in BEAS2B cells. (b) Similar results were observed following 48-h exposure to both compounds. The upper right inset compares each compound to complete cell lysis and LDH release with 2% Triton (100% cell death). (c) Again, no detectable toxicity was determined in primary human upper airway epithelial cells under submersion conditions following 96-h exposure to both compounds as measured by LDH release. (d) The same cultures were further evaluated following prolonged exposure to bpV(phen) and demonstrate sustained phosphorylation of Akt above baseline levels in BEAS2B cells. Data are mean±s.d.; n=3 with a representative western blot.
Examination of compound specificity
To confirm that Akt phosphorylation was specifically induced following PTEN inhibition, a PTEN-positive cell line, DU145, and two PTEN-negative cell lines, LNCaP and PC3, were first examined with bpV(phen). The DU145 as well as BEAS2B cells demonstrated a dose-responsive increase in Akt phosphorylation (Figures 1b and 3a), whereas the PTEN-negative cell lines, LNCaP and PC3, showed no evidence of Akt phosphorylation over the same dosage range (Figures 3b and c). This demonstrated that bpV(phen) specifically inhibits PTEN, which in turn is directly responsible for Akt phosphorylation.
Figure 3.
Evaluation of the specificity of bisperoxovanadium compounds for PTEN (phosphatase and tensin homologue deleted on chromosome ten). (a) A dose-dependent increase in Akt phosphorylation was observed with potassium bisperoxo (1,10-phenanthroline) oxovanadate (bpV(phen)) in DU145 cells, a PTEN functional cell line. The dosage range evaluated was between 1 and 5 μM for 0.5 h. Phosphorylated Akt and total Akt levels are shown following western analysis (top panel) and densitometry (bottom panel). (b) We then determined if a similar dose-dependent increase in Akt phosphorylation would be observed with bpV(phen) over an identical dose range in the PTEN-null LNCaP or (c) PC3 cell lines. As before, phosphorylated Akt and total Akt levels were evaluated by western analysis (top panel) and densitometry (bottom panel). In contrast to DU145 cells, bpV(phen) did not induce Akt phosphorylation in PTEN-null cells. The results shown are mean±s.d.; n=3, with representative western blots.
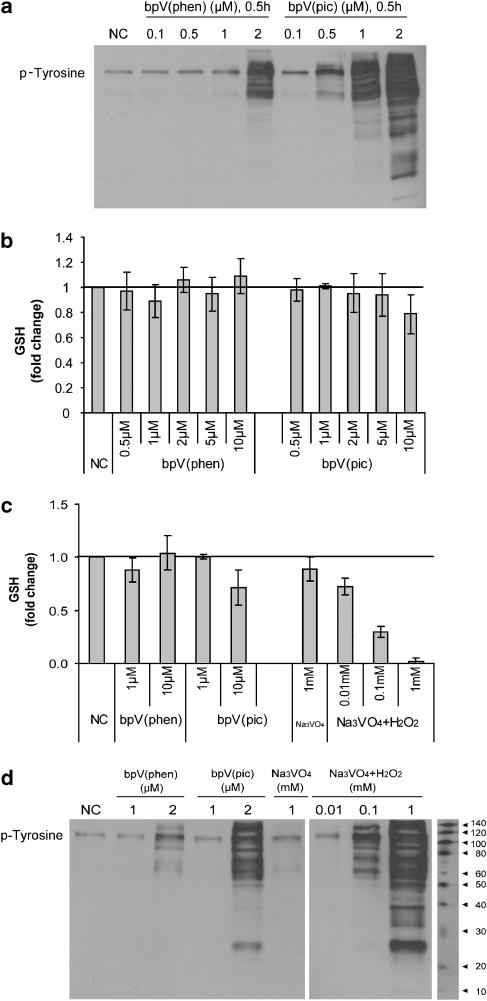
To further validate specificity of the inhibitors, we examined whole-cell lysates for evidence of phosphotyrosine residues by western blotting. Previous studies utilizing the original parent vanadium compounds showed a substantial increase in protein tyrosine phosphorylation as a consequence of their broad reactivity towards multiple protein tyrosine phosphatases. We observed similar results when vanadate was compared to the bisperoxovanadium compounds. Presumably, if bpV(phen) and bpV(pic) are more specific for the intended target, we would observe significantly less phosphotyrosine staining. bpV(phen) exposure for 30 min at a dose of 0.1–1 μM showed no evidence of phosphotyrosine residues, whereas exposure to 2 μM showed a significant increase in phosphotyrosine staining. In the case of bpV(pic), a substantial increase in phosphotyrosine residues was observed at lower doses starting at 0.5 μM and was further increased at 1 and 2 μM (Figure 4a) In addition, we conducted a time-course exposure with bpV(phen) for up to 24 h at 1 μM. No increase in anti-phosphotyrosine staining was observed within the time frame examined (data not shown), suggesting that bpV(phen) is relatively specific for PTEN and not other tyrosine phosphatases at a concentration of 1 μM or lower. In comparison, bpV(pic) is less specific for the intended target and appears to interact with other targets, presumably phosphatases, at similar drug concentrations.
Figure 4.
Bisperoxovanadium compounds are specific for PTEN (phosphatase and tensin homologue deleted on chromosome ten) in lung epithelia. (a) BEAS2B cultures were exposed to potassium bisperoxo (1,10-phenanthroline) oxovanadate (bpV(phen)) and di-potassium bisperoxo (picolinato) oxovanadate (bpV(pic)) at a dosage range between 0.1 and 2 μM for 0.5 h. Whole-cell lysates were resolved on a polyacrylamide gel and then evaluated for phosphotyrosine content by western analysis. By direct comparison, bpV(phen) was superior to bpV(pic) in maintaining target specificity over a broader dosage range. (b) In similar experiments, we evaluated the potential for a dose-dependent increase in glutathione content, indicative of a lack of PTEN specificity, over a broader dosage range (0.5–10 μM) for bpV(phen) and bpV(pic) in BEAS2B cultures. Cell lysates were collected after 0.5-h exposure and measured for glutathione content. Again, by direct comparison, bpV(phen) was superior to bpV(pic) in maintaining target specificity over a broader dosage range. (c) As a positive control, we also compared bpV(phen) and bpV(pic) to the less-specific parent compound Na3VO4 following activation with H2O2 (0.01–1 mM) for 0.5 h. H2O2-activated Na3VO4 resulted in a substantial decrease in glutathione content, indicative of broad substrate inhibition. (d) Similarly, a dose-responsive increase in phospho-tyrosine residues as measured by anti-phosphotyrosine staining was observed under the same experimental conditions. Data are mean±s.d.; n=3, with representative Western blots.
Intracellular glutathione content served as a second measure to determine PTEN specificity. At a dose between 0.5 and 10 μM for both bpV(phen) and bpV(pic), no change in glutathione concentration was observed with bpV(phen) treatment. However, at a 10 μM dose for bpV(pic), glutathione depletion was observed, indicating loss of specificity with this compound (Figure 4b). This result is consistent with phosphotyrosine analysis in that bpV(pic) became less specific for PTEN at higher doses. Sodium orthovanadate, a peroxovanadium compound known to be less specific for PTEN, served as a positive control for comparison to both compounds. A dose-responsive depletion of glutathione was observed with sodium orthovanadate, thereby validating the assay and demonstrating the relative specificity of the bisperoxovanadium compounds for PTEN (Figure 4c). Consistent with the above findings, a dose-responsive increase in phosphotyrosine residues was also observed with sodium orthovanadate (Figure 4d).
Enhanced wound closure by PTEN inhibitors
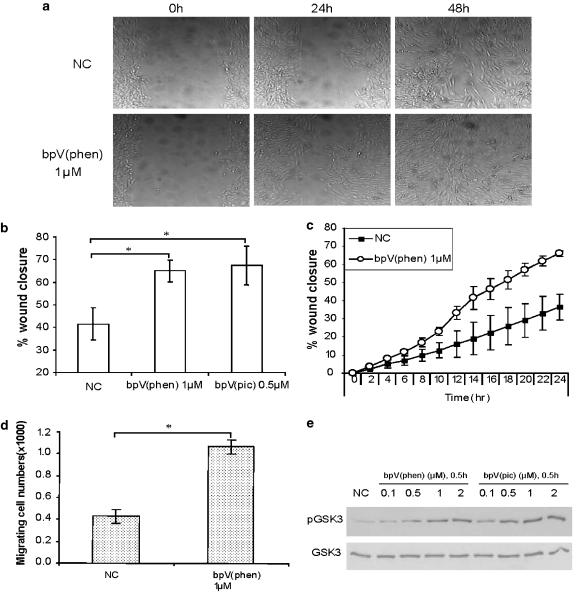
Next, we evaluated a mechanical ‘scrape wound' injury model in lung epithelial cells to determine if PTEN inhibition with bisperoxovanadium compounds enhances wound closure. We observed consistent and accelerated wound closure across the entire area following bpV(phen) and bpV(pic) treatment at low micromolar concentrations (1 and 0.5 μM, respectively) within 24 h in BEAS2B cells (Figure 5a) and primary cultures (shown later in Figure 8a). Furthermore, re-establishment of an intact monolayer occurred in a statistically significant shorter period of time in the treatment groups when compared to untreated controls (Figure 5b). Time-course analysis of wound closure, with or without exposure to bpV(phen) (1 μM), demonstrated that accelerated wound closure was evident at early time points, suggesting that cell migration plays a prominent role in the early stages of wound recovery in this model (Figure 5c). In support of this, evaluation of cell migration demonstrated a statistically significant increase in migrating cell numbers following exposure to bpV(phen) 1 μM (P<0.05) (Figure 5d). A concomitant, dose-responsive increase in GSK3 phosphorylation by both bpV(phen) and bpV(pic) was observed, thereby providing preliminary evidence of key downstream intermediates involved in wound closure enhancement following PTEN inhibition (Figure 5e).
Figure 5.
Bisperoxovanadium compounds enhance wound closure in the lung epithelium. (a) A 600 μM scrape wound was made in confluent BEAS2B monolayers immediately followed by addition of PTEN (phosphatase and tensin homologue deleted on chromosome ten) inhibitors to cultures. Microscopic images of cultures were obtained over a 48-h period utilizing time-lapse photomicroscopy of live cell cultures. Images shown are of non-treated control or potassium bisperoxo (1,10-phenanthroline) oxovanadate (bpV(phen))-treated cultures at 0 h (left panel), 24 h (middle panel) and 48 h (right panel). Similar images were obtained with both compounds in primary lung epithelial cultures (shown later in Figure 8). (b) Quantitative analysis of wound closure following treatment with PTEN inhibitors in BEAS2B cells demonstrates that 24-h exposure to both bisperoxovanadium compounds resulted in accelerated closure of the lesion when compared to untreated cultures. Wound widths were initially measured immediately after making the lesion (0 h) (100% distance) and then at 24 h after treatment to determine the distance remaining between wound edge margins. Multiple time points were analysed, but only the 24-h data are shown. The percentage of wound closure was then calculated and presented graphically. Both bpV(phen) (1 μM) and di-potassium bisperoxo (picolinato) oxovanadate (bpV(pic)) (0.5 μM) induced a statistically significant enhancement in wound closure when compared to untreated cultures. Data are mean±s.d.; n=3; *P<0.05 vs NC; Student's t-test. (c) Time course of wound closure with or without exposure to bpV(phen) (1 μM) demonstrates accelerated wound closure at early time points, suggesting that cell migration plays a role in wound healing in this model. Multiple time points were analysed every 2 h for 24 h. The percentage of wound closure was calculated as described previously. Data are mean±s.d.; n=3. (d) A migration assay was also utilized to evaluate cell behaviour following exposure to bpV(phen) (1 μM). BEAS2B cells were plated on the apical of migration chamber with or without treatment and incubated at 37 °C to allow migration. Cells that migrated onto the basolateral surface were then dissociated at designated time points and counted. Data are mean±s.d.; n=3; *P<0.05 vs NC; Student's t-test. (e) A simultaneous dose-dependent increase in phospho-glycogen synthase kinase-3 (GSK3), a downstream target of Akt, was observed following 24-h treatment with bpV(phen) or bpV(pic) in BEAS2B cultures. Phospho-GSK3 and total GSK3 levels were analysed by western analysis.
Enhanced wound closure by PTEN suppression
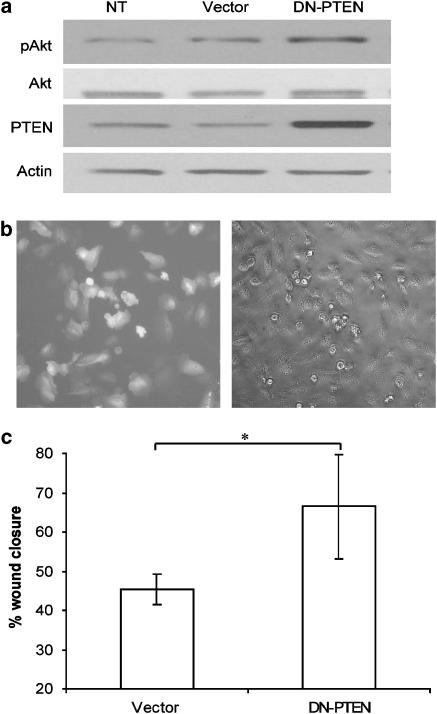
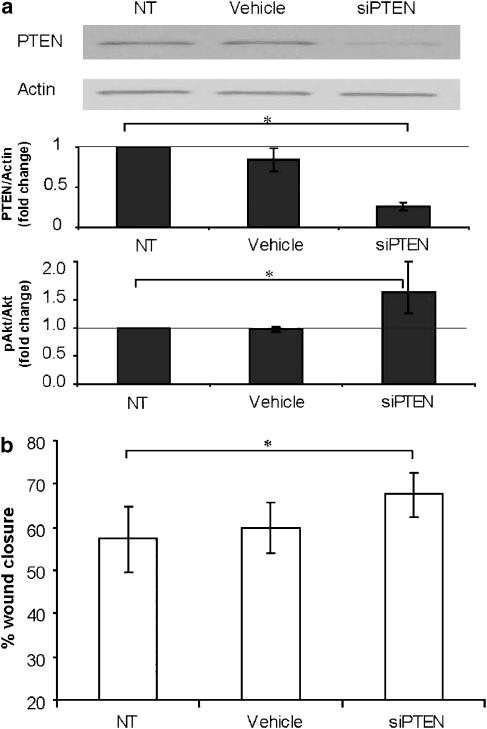
To further validate the role of PTEN in epithelial wound repair, we first conducted loss-of-function studies with transient transfection of a plasmid expressing a DN form of PTEN (DN-PTEN) in BEAS2B cells. Increased Akt phosphorylation was observed following transfection with the DN-PTEN plasmid (Figures 6a and b). DN-PTEN-transfected cells also demonstrated a significant enhancement in wound closure, although with a greater degree of variation across the wound margin (Figure 6c), when compared to the vector-only control transfection group, thereby further supporting PTEN as a participant in wound repair. We then conducted a complementary study utilizing siRNA-mediated suppression of PTEN and again evaluated its impact on the wound closure rate. We were able to routinely achieve a 70% decrease in PTEN protein expression at 48 h after siRNA delivery, which resulted in an almost twofold increase in Akt phosphorylation, thereby indicating successful PTEN gene suppression (Figure 7a). Similar to the previous results with DN-PTEN transfection, siRNA-mediated suppression of PTEN resulted in a statistically significant acceleration in wound closure rate when compared to the control group (Figure 7b). These results provided additional mechanistic evidence to validate PTEN as a molecular target involved in repair following injury to the lung epithelium.
Figure 6.
Overexpression of dominant negative (DN) PTEN (phosphatase and tensin homologue deleted on chromosome ten) enhances wound closure in lung epithelia. To determine further if PTEN plays a critical role in wound closure, we transiently overexpressed a DN form of PTEN and examined wound closure rate. (a) Overexpression of DN-PTEN resulted in an increase in PTEN levels and a simultaneous increase in phosphorylated Akt levels in BEAS2B cultures. Total Akt and β-actin levels were unaffected. (b) A transfection efficiency of 70% or greater was routinely achieved as shown by expression of the green fluorescent protein reporter plasmid at 24 h after transfection. (c) Quantitative assessment of wound closure is shown following transfection with the DN-PTEN plasmid. Scrape wounds were made 24 h after transfection and then images were analysed 24 h later. A statistically significant enhancement in wound closure was observed at 24 h in DN-PTEN-transfected cultures when compared to sham-transfected cultures (empty vector). Data are mean±s.d.; n=3; *P<0.05 vs vector; Student's t-test.
Figure 7.
Loss of function with PTEN (phosphatase and tensin homologue deleted on chromosome ten) siRNA knockdown enhances wound closure in lung epithelia. To confirm the role of PTEN as a suppressor in lung epithelial wound repair, we utilized siRNA directed at PTEN in scrape wound cultures. (a) Confirmation of successful siRNA-mediated suppression of PTEN protein expression was first done in BEAS2B cultures 48 h after transfection. PTEN protein levels were determined by western analysis (top panel) and measured by densitometry and standardized to β-actin (middle panel). The level of phosphorylated Akt was also evaluated in the same cultures and presented as the ratio of phosphorylated Akt to total Akt (bottom panel). (b) We then evaluated wound closure in cultures following siRNA-mediated PTEN knockdown. Scrape wounds were made 48 h after transfection and images were analysed 24 h later. Similar to previous experiments, a statistically significant enhancement in wound closure was observed following PTEN suppression when compared to untreated or vehicle-treated cultures. Data are mean±s.d.; n=3; *P<0.05 as shown; Student's t-test.
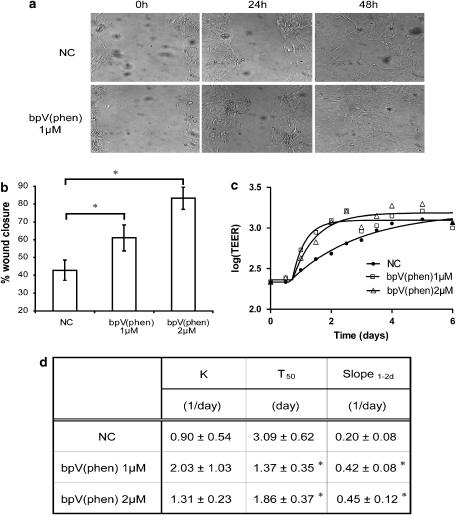
Enhanced wound closure by PTEN inhibitors in hUAECs
Next, we exposed primary human upper airway epithelia to both compounds under two different culture conditions that included conventional submersion cultures as well as fully differentiated cultures grown at an air-to-liquid interface on transwell inserts. Exposure to both bisperoxovanadium compounds in primary hUAECs grown under submersion conditions resulted in a significant enhancement in wound closure (Figures 8a and b). In parallel studies with differentiated cultures, we observed that both compounds also induced a faster recovery of monolayer integrity, as measured by increasing TEER. As shown, re-establishment of monolayer TEER (recovery) occurred in a shorter period of time with bpV(phen) at doses of 1 and 2 μM when compared to the non-treated control group (Figure 8c). Kinetic analysis demonstrated higher recovery rate, shorter halftime of recovery (T50) and a steeper slope over 1–2 days (slope1−2d) at both doses over the 6-day monitoring interval (Figure 8d), suggesting that both bisperoxovanadium compounds demonstrate therapeutic potential to enhance wound closure and restore lung epithelial monolayer integrity following injury. Similar results were obtained with bpV(pic) (data not shown).
Figure 8.
Bisperoxovanadium compounds enhance wound closure in primary human upper airway epithelial cells (hUAECs). (a) A 600 μM scrape wound was made in confluent primary, submersed, cell cultures immediately followed by addition of PTEN (phosphatase and tensin homologue deleted on chromosome ten) inhibitors to cultures. Microscopic images of cultures were obtained over a 48-h period. Images are shown of non-treated control or potassium bisperoxo (1,10-phenanthroline) oxovanadate (bpV(phen))-treated cultures at 0 h (left panel), 24 h (middle panel) and 48 h (right panel). (b) Quantitative analysis of wound closure following treatment with PTEN inhibitors in primary cells under submersion conditions demonstrates that 24-h exposure to both bisperoxovanadium compounds resulted in accelerated closure of the lesion when compared to untreated cultures. The results shown were obtained following measurement of bpV(phen)-treated cultures. Similar results were observed with di-potassium bisperoxo (picolinato) oxovanadate (bpV(pic)) at the same doses (not shown). Again, a statistically significant difference in wound closure was observed following treatment with PTEN inhibitors. Data are mean±s.d.; n=3; *P<0.05 vs NC; analysis of variance. (c) Serial measurements of trans-epithelial electrical resistance (TEER) was also monitored out to 6 days after a mechanical wound was generated in fully differentiated primary hUAECs grown on transwell inserts. In parallel, we compared cultures without any treatment to those receiving bpV(phen) at a dose of 1 and 2 μM. (d) Metric analysis of TEER recovery is expressed by K, TEER recovery rate constant, T50, time to 50% recovery as well as slope1−2d, the linear section of the curve. Data are mean±s.d.; n=3; *P<0.05 vs NC; analysis of variance.
Discussion
PTEN, an inositol polyphosphate 3-phosphatase, was first identified as a tumour suppressor that becomes inactivated in glioblastomas leading to tumour formation (Li et al., 1997). Deletion or inactivation of the gene encoding PTEN has since been observed to play a similar role in the development of other forms of cancer (Cairns et al., 1997; Dahia et al., 1997; Guldberg et al., 1997; Tashiro et al., 1997). More recently, PTEN has been identified as a negative regulator of insulin-dependent signalling and PTEN depletion restored insulin responsiveness and normalized glucose metabolism in diabetic animals (Butler et al., 2002). This has raised interest in bisperoxovanadium compounds as therapeutic insulin-mimetic agents (Vinciguerra and Foti, 2006). Related to this, it is also known that PTEN regulates cell migration and apoptosis (Vazquez and Sellers, 2000; Vazquez et al., 2000). Therefore, through its opposing effects on cell signalling, PTEN has the ability to antagonize both growth-promoting and antiapoptotic pathways. Collectively, these observations indicate that a complete loss of PTEN activity is detrimental to the host, whereas controlled suppression has the potential to provide therapeutic benefit.
The essential biochemical role of PTEN is to remove the D3 phosphate from the inositol ring of phosphoinositides (Maehama and Dixon, 1998). The PTEN-active site pocket is larger than other protein phosphatases, allowing both bulky and smaller phosphoamino acids to be substrates. However, due to the unique physical attributes within the reactive site, electrostatic interactions with positively charged side chains, hydrogen bonds and polar side chains are favoured (Lee et al., 1999). As a result, the preferred in vivo substrate for PTEN is PI3K-generated PI(3,4,5)P3. Interaction between PI(3,4,5)P3 and PTEN leads to inactivation of the PI3K signalling axis, thereby preventing phosphorylation of the downstream intermediate, Akt.
In view of our previous work and others (Ray et al., 2003; Bao et al., 2005; Ray, 2005), we hypothesized that transient activation of the PI3K/Akt signalling axis would facilitate epithelial wound repair. In this investigation, we focused our attention on PTEN, the primary suppressor of PI3K/Akt signalling. The two objectives of our study were to assess PTEN as a molecular target in lung epithelial wound repair and evaluate the in vitro therapeutic potential of two bisperoxovanadium compounds, known inhibitors of PTEN phosphatase activity. At the molecular level, our findings revealed that PTEN is relatively abundant in the lung epithelium and that suppression of the endogenous protein stimulates wound healing. We also observed that two peroxovanadium compounds, bpV(phen) and bpV(pic), were effective inhibitors of PTEN resulting in increased phosphorylation of Akt in lung epithelia. In direct comparison, bpV(phen) was superior to bpV(pic) in that target specificity was maintained over a broader and lower submicromolar dosage range associated with minimal cellular toxicity. Most strikingly, our results demonstrate that inhibition of PTEN with both the agents significantly enhances epithelial wound closure. The exact mechanism to explain these findings remains unclear but likely involves effects on cell migration and possibly cell proliferation. This is not surprising considering that PTEN has been reported to block cell cycle progression through its lipid phosphatase activity (Weng et al., 2001) and that overexpression of PTEN has been reported to suppress cell migration (Saga et al., 2003). To begin to understand the mechanism(s) for wound closure enhancement in our model, we conducted a cell migration assay following bpV(phen) treatment and concomitantly evaluated cell proliferation. Our results indicate that cell migration accounted for a majority of wound closure enhancement, whereas evidence in support of cell proliferation was not observed. Further investigations that more thoroughly evaluate the role of the bisperoxovanadium compounds on cell migration and proliferation are warranted.
The PI3K/phosphoinositide-dependent protein kinase 1/Akt signalling cascade is the immediate converging point for many receptor tyrosine kinase- and cytokine-mediated pathways that regulate cell growth and proliferation (Chan et al., 1999; Datta and Lianos, 1999). In the early stages of this signalling pathway, growth factor-mediated activation of PI3K results in an increase in lipid products PI(3,4,5)P3 and phosphatidylinositol 3,4-bisphosphate in the plasma membrane (Franke et al., 1997). These PI3K lipid products then bind Akt via its pleckstrin homology domain, and induce the membrane co-translocation of Akt with phosphoinositide-dependent protein kinase 1, thereby facilitating phosphorylation and activation of Akt by phosphoinositide-dependent protein kinase 1. Akt then mediates its effect by phosphorylating downstream targets, including glycogen synthase-3β, that are involved in mediating cell migration, proliferation and survival (Cantley, 2002). This biochemical cascade provides a framework to account for the ability of bpV(phen) and bpV(pic) to facilitate epithelial wound closure. In studies utilizing fully differentiated primary human lung epithelia and related cell line cultures, we observed that PTEN is constitutively abundant. We also consistently detected the presence of low levels of phosphorylated Akt. The mechanical scrape wound model did not use an extrinsic stimulus to provoke PI3K activity; therefore, we interpret these findings to suggest that PTEN is a constant suppressor of PI(3,4,5)P3-mediated downstream signalling events in lung epithelia. Furthermore, the relatively potent and specific PTEN inhibitors used in our studies were able to readily penetrate the epithelium and inhibit PTEN, thereby ‘taking the brake off' this pathway and allowing PI(3,4,5)P3 to more readily activate its intended target. In view of these findings, it becomes reasonable to hypothesize that inflammation in the microenvironment of the airway, in which known activators of the PI3K axis are enriched, may synergize the ability of these compounds to facilitate wound closure. Also, our findings obtained with primary cultures suggest that this class of compounds may be well suited for in vivo topical delivery strategies that are designed to locally maximize drug concentration in epithelia, enhance wound repair and minimize collateral adverse effects, although this remains to be tested.
It is known that bisperoxovanadium compounds with polar side chains, including bpV(phen) and bpV(pic), have a strong preference for PTEN (IC50; 20–40 nM) with minimal cellular toxicity, whereas the parent compound vanadate is a promiscuous inhibitor of several other phosphatases and possesses a broader array of effects, perhaps some that are undesirable (Posner et al., 1994; Schmid et al., 2004). Our findings support these conclusions. This along with their relatively straightforward structure that is amenable to further manipulation, and known stability in both powder and solution, make the bisperoxovanadium compounds attractive for further consideration as topically administered therapeutic agents to prevent or facilitate epithelial wound repair in the lung. In view of the natural half-life of PTEN (48–72 h) (Wu et al., 2000) and the relatively short half-life of these irreversible inhibitors, one can predict that inhibition of PTEN could be sustained over a suitable period of time until PTEN levels are replenished by the cell, thereby providing a temporary, inhibitory effect. When considering these attributes and the central role of epithelial damage in lung pathogenesis, a number of different diseases may benefit from PTEN inhibition, as demonstrated here.
Acknowledgments
Support from the National Institutes of Health (KO8 HL56336) and (R01 HL086981-01) (DLK) and The American College of Clinical Pharmacy (CIA) (DLK) is gratefully acknowledged. We owe special thanks to Lifeline of Ohio Tissue Procurement Agency.
Abbreviations
- bpV(phen)
potassium bisperoxo (1,10-phenanthroline) oxovanadate
- bpV(pic)
di-potassium bisperoxo (picolinato) oxovanadate
- DN
dominant negative
- GSK3
glycogen synthase kinase-3
- hUAECs
primary human upper airway epithelial cells
- LDH
lactate dehydrogenase
- PBS
phosphate-buffered solution
- PI(3,4,5)P3
phosphatidyl-inositol 3,4,5-trisphosphate
- PI3K
phosphoinositide-3 kinase
- PTEN
phosphatase and tensin homologue deleted on chromosome ten
- WST-1
water-soluble tetrazolium salt
Conflict of interest
The authors state no conflict of interest.
References
- Bao S, Wang Y, Sweeney P, Chaudhuri A, Doseff AI, Marsh CB, et al. Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L36–L42. doi: 10.1152/ajplung.00309.2003. [DOI] [PubMed] [Google Scholar]
- Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cerovac Z, Ban J, Morinville A, Yaccato K, Shaver A, Maysinger D. Activation of MAPK by potassium bisperoxo(1,10-phenanthroline)oxovanadate (V) Neurochem Int. 1999;34:337–344. doi: 10.1016/s0197-0186(99)00018-2. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Dahia PL, Marsh DJ, Zheng Z, Zedenius J, Komminoth P, Frisk T, et al. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res. 1997;57:4710–4713. [PubMed] [Google Scholar]
- Datta PK, Lianos EA. Retinoic acids inhibit inducible nitric oxide synthase expression in mesangial cells. Kidney Int. 1999;56:486–493. doi: 10.1046/j.1523-1755.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST.Airway remodeling in asthma: new insights J Allergy Clin Immunol 2003111215–225.quiz 226 [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997;57:3660–3663. [PubMed] [Google Scholar]
- Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, et al. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol. 2006;30:34–43. doi: 10.1053/j.semperi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Lu Y, Parkyn L, Otterbein LE, Kureishi Y, Walsh K, Ray A, et al. Activated Akt protects the lung from oxidant-induced injury and delays death of mice. J Exp Med. 2001;193:545–549. doi: 10.1084/jem.193.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol. 2005;289:L685–L695. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- Posner BI, Faure R, Burgess JW, Bevan AP, Lachance D, Zhang-Sun G, et al. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem. 1994;269:4596–4604. [PubMed] [Google Scholar]
- Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–733. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- Ray P, Devaux Y, Stolz DB, Yarlagadda M, Watkins SC, Lu Y, et al. Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proc Natl Acad Sci USA. 2003;100:6098–6103. doi: 10.1073/pnas.1031851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. Protection of epithelial cells by keratinocyte growth factor signaling. Proc Am Thorac Soc. 2005;2:221–225. doi: 10.1513/pats.200502-012AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumora L, Hadzija M, Maysinger D, Zanic-Grubisic T. Positive regulation of ERK activation and MKP-1 expression by peroxovanadium complex bpV (phen) Cell Biol Toxicol. 2004;20:293–301. doi: 10.1007/s10565-004-5104-5. [DOI] [PubMed] [Google Scholar]
- Saga Y, Mizukami H, Takei Y, Ozawa K, Suzuki M. Suppression of cell migration in ovarian cancer cells mediated by PTEN overexpression. Int J Oncol. 2003;23:1109–1113. [PubMed] [Google Scholar]
- Schmid AC, Byrne RD, Vilar R, Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Tamura M, Gu J, Takino T, Yamada KM. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res. 1999;59:442–449. [PubMed] [Google Scholar]
- Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Sellers WR. The PTEN tumor suppressor protein: an antagonist of phosphoinositide 3-kinase signaling. Biochim Biophys Acta. 2000;1470:M21–M35. doi: 10.1016/s0304-419x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Vinciguerra M, Foti M. PTEN and SHIP2 phosphoinositide phosphatases as negative regulators of insulin signalling. Arch Physiol Biochem. 2006;112:89–104. doi: 10.1080/13813450600711359. [DOI] [PubMed] [Google Scholar]
- Weng LP, Brown JL, Eng C. PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet. 2001;10:599–604. doi: 10.1093/hmg/10.6.599. [DOI] [PubMed] [Google Scholar]
- Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye MB, Yuan XJ, et al. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci USA. 2000;97:4233–4238. doi: 10.1073/pnas.97.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]