Abstract
Morphological responses of plants to shading have long been studied as a function of light quality, in particular the ratio of red to far red light that affects phytochrome activity. However, changes in light quantity are also expected to be important for the shading response because plants have to adapt to the reduction in overall energy input. Here, we present data on the involvement of auxin and ethylene in the response to low light intensities. Decreased light intensities coincided with increased ethylene production in Arabidopsis rosettes. This response was rapid because the plants reacted within minutes. In addition, ethylene- and auxin-insensitive mutants are impaired in their reaction to shading, which is reflected by a defect in leaf elevation and an aberrant leaf biomass allocation. On the molecular level, several auxin-inducible genes are up-regulated in wild-type Arabidopsis in response to a reduction in light intensity, including the primary auxin response gene IAA3 and a protein with similarity to AUX22 and the 1-aminocyclopropane-1-carboxylic acid synthase genes ACS6, ACS8, and ACS9 that are involved in ethylene biosynthesis. Taken together, the data show that ethylene and auxin signaling are required for the response to low light intensities.
One of the more important environmental factors for plants is the availability of sufficient light. Shading in nature consists of distinguishable features. First, there are the changes in light quality, most often an increase in far red (FR) light due to neighboring vegetation or end of day effects. Within canopies, other spectral changes are also observed (Holmes, 1983). Second, there is a diminishment of light intensity or photosynthetic photon flux density (PPFD), including blue and red (R) light, which has direct effects on photosynthesis and photomorphogenesis. Although a large amount of data is available on the influence of light intensity on general biomass production and root to shoot partitioning (Blackman and Wilson, 1951; McConnaughay and Coleman, 1999), relatively little is known about more subtle effects of light quantity on plant architecture and morphogenesis. The latter effects include biomass allocation within organs and leaf form.
Many plant species try to avoid shading and adapt their phenotype to reach out for light (Holmes, 1983). Rosette plants can orient their leaves to a more vertical position, sometimes referred to as hyponasty, and redirect accumulation of biomass to stems and petioles rather than leaf blades (Smith, 1992; Hangarter, 1997; Maliakal et al., 1999).
GAs, ethylene, or auxins can induce reorientation of leaves (Brock et al., 1994; Clua et al., 1996; Cox et al., 2003). In leaf blades, hyponasty can result from auxin-induced differential growth (Lippincott and Lippincott, 1971). In Arabidopsis, asymmetric distribution of auxins causes gravitropic and phototropic responses (Friml et al., 2002). A number of Arabidopsis auxin mutants, called massugu (msg1/nph4/arf7), have constitutive nastic responses, which cause upward or downward orientation of leaves (Watahiki and Yamamoto, 1997; Harper et al., 2000). Auxin is not solely responsible for differential growth but can also interact with ethylene (Lehman et al., 1996; Luschnig et al., 1998; Müller et al., 1998 Harper et al., 2000; Friml et al., 2002). In certain cases, as in the development of the apical hook in etiolated Arabidopsis seedlings, the redistribution of auxins is stimulated by ethylene. The hls1-1 (hookless1-1) mutant is impaired in this process (Lehman et al., 1996). In tomato (Lycopersicon esculentum), auxin and ethylene also interact in gravitropic and epinastic responses (Ursin and Bradford, 1989; Madlung et al., 1999; Hansen and Grossmann, 2000).
PhyB (Phytochrome B) mutants have elevated petioles even in non-shaded conditions (Somers et al., 1991). In addition, their hypocotyls and petioles are elongated, and they display an increased apical dominance, as seen in shaded wild-type plants (Koornneef et al., 1980; Reed et al., 1993; Devlin et al., 1996). These responses also can be induced by auxins (Smalle et al., 1997; Chatfield et al., 2000; Sawa et al., 2002). In a screen for suppressors of phytochrome mutants, shy2, a mutant defective in the auxin response gene IAA3, was isolated (Kim et al., 1996; Tian and Reed, 1999). Furthermore, a number of AUX/IAA proteins can be phosphorylated by phytochrome A (Colon-Carmona et al., 2000). Thus, the light signaling and auxin pathways are clearly intertwined.
A first indication for a correlation between ethylene action and hyponasty was presented in a study of the submergence response of Rumex palustris. In this flooding-tolerant species, ethylene induces hyponasty and extension of petioles, allowing the leaf blades to reach the water surface (Cox et al., 2003).
On the other hand, there is also a relation between light signaling and ethylene. Sorghum (Sorghum bicolor) wild-type plants subjected to dim FR-enriched light, and phyB mutants produced more ethylene than wild-type plants under white light (Finlayson et al., 1998). Together, these phenotypic similarities indicate a possible role for ethylene in a plant's response to shading.
Data on ethylene and light interactions in vegetative development are rather scarce. It is generally accepted that light inhibits ethylene synthesis by reducing 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase (ACO) activity (Kao and Yang, 1982; Finlaysonet al., 1998). In leaf segments of oat (Avena sativa) and Begonia hiemalis-Fotsch, it was demonstrated that light quality also has an influence on ethylene production (Rudnicki et al., 1993; Corbineau et al., 1995).
Considering the phenotypic overlaps of shade, auxin, and ethylene responses, we decided to investigate to which extent these factors work together to determine plant architecture. In the majority of previous studies on shading, the emphasis was put on qualitative differences, i.e. changes in the R to FR ratio. Nevertheless, reacting to mere changes in light intensity can be of considerable importance in pioneer plants such as Epilobium species and Arabidopsis (Chapin et al., 1994; Pigliucci and Schmitt, 1999; Al Shehbaz and O'Kane, 2002). A typical strategy for survival in this type of plant species is the production of a large number of small seeds. As a consequence, seedlings are small and often exposed to shading caused by irregularities of the soil surface. Here, we present evidence for the involvement of both auxin and ethylene in the developmental response to decreased light intensity.
RESULTS
Low Light Intensity Is Correlated with an Increase in Ethylene Production
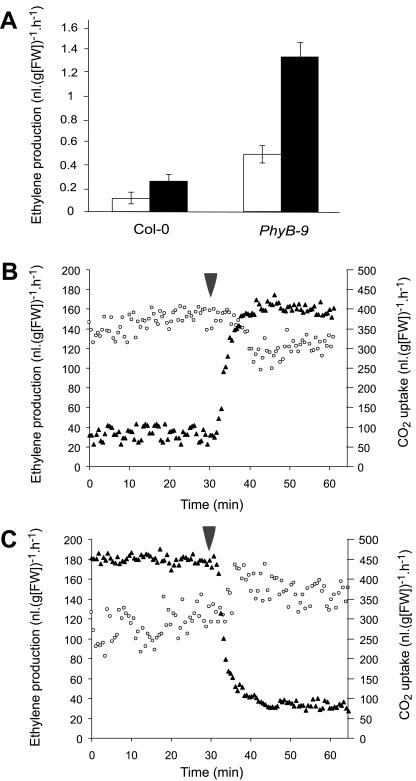
Ethylene production of intact 2-week-old Arabidopsis seedlings was measured using laser-based photo-acoustic detection, able to detect levels as low as 1 nL L–1 (Bijnen et al., 1996). After accumulation in a closed cuvette, higher production was found in wild-type plants grown in low light, compared with plants grown in high light (Fig. 1A). PhyB-9 mutant seedlings showed the same trend but overproduced ethylene in both conditions as compared with wild type (Fig. 1A).
Figure 1.
Ethylene production in different light intensities. A, Ethylene production from 2-week-old Arabidopsis wild-type and mutant phyB-9 rosettes. Plants were kept in gas-tight vials for 2.5 h. Accumulated ethylene was measured using photo-acoustic detection. White bars = 125 μmol m–2 s–1 PPFD. Black bars = 35 μmol m–2 s–1 of PPFD. Error bars = se. B, Changes in ethylene production in ACC-treated rosettes upon a switch in light intensity from 30 to 125 μmol m–2 s–1 PPFD. Measurements were performed online in 2-week-old plants on medium containing 50 μm ACC. Black triangles, CO2 uptake; white circles, emanated ethylene. Arrowhead, Time point of light switch. FW, Fresh weight. B and C, Changes in ethylene production in ACC-treated rosettes upon a switch in light intensity from 125 to 30 μmol m–2 s–1 PPFD. Measurements were performed online in 2-week-old plants on medium containing 50 μm ACC. Black triangles, CO2 uptake; white circles, emanated ethylene. Arrowhead, Time point of light switch. FW, Fresh weight.
Plants grown on medium containing the ethylene precursor ACC produced sufficient amounts of ethylene for continuous flow detection. To measure changes in ethylene production in Arabidopsis upon a switch in light intensity, seedlings were grown on a saturating concentration of ACC (50 μm; Smalle et al., 1997). This concentration of ACC was used to prevent interference of varying activities of ACC synthases (ACSs) that control a rate-limiting step for ethylene biosynthesis. In these conditions, having a continuous supply of its substrate ACC, ACO activity limits ethylene production. Switching from low light intensity (30 μmol m–2 s–1) to a higher light intensity (130 μmol m–2 s–1) inhibited ethylene production within minutes. The change in ethylene production coincided with an increase in CO2 uptake, indicating that the change in ethylene emanation is not due to stomatal closure (Fig. 1B). Switching from high light to low light intensity caused the opposite effect (Fig. 1C). Addition of extra FR light, without altering the PPFD of 130 μmol m–2 s–1, but causing a drop in R:FR ratio from 2.25 to 0.67, did not increase ethylene production, as compared with plants that remained in light with 130 μmol m–2 s–1 PPFD and a R:FR ratio of 2.25 (data not shown).
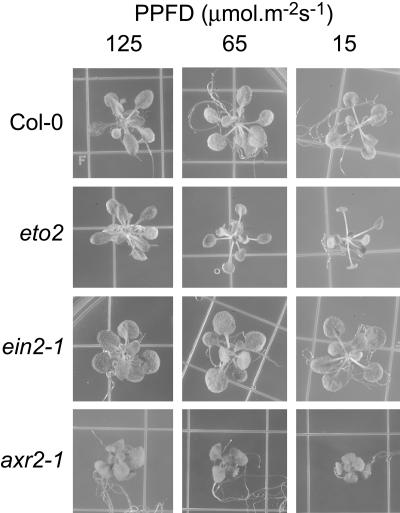
Effects of Light Intensity on Leaf Elevation Angles in Ethylene and Auxin Mutants
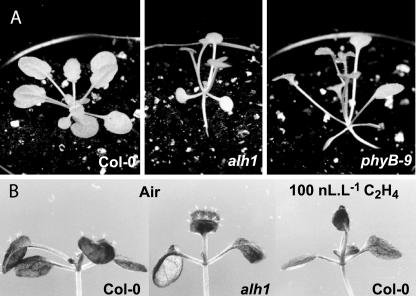
Auxin-insensitive mutants are affected in numerous developmental processes (for review, see Tian and Reed, 2001; Leyser, 2002; Swarup et al., 2002). Of particular interest are the massugu1 (msg1/nph4/ARF7) mutants. In general, they show leaf epinasty; however, one-half of the plants of the msg1-3 mutant line have hyponastic leaves (Watahiki and Yamamoto, 1997). We have characterized the alh1 (ACC-related long hypocotyl 1) mutant that shows both ethylene- and auxin-related phenotypes (Vandenbussche et al., 2003). At light intensities below 50 μmol m–2 s–1 PPFD, leaves of this mutant point upwards, in contrast to wild-type leaves that displayed a more horizontal growth. As a consequence, the alh1 phenotype in these conditions was highly similar to phyB plants (Fig. 2A). This phenotype could also be mimicked by treatment of wild-type seedlings with exogenous ethylene for 6 d (Fig. 2B), preventing horizontal leaf positioning.
Figure 2.
A, Alh1 has a larger leaf elevation angle. Left to right, Wild-type Col-0, alh1, and PhyB-9. Plants were grown for 33 d on soil at a PPFD of 30 μmol m–2 s–1. B, Treatment of wild-type Arabidopsis with ethylene keeps leaves vertically oriented and phenocopies alh1. Left, Air-treated alh1; middle, air treated wild type Col-0; right, ethylene-treated wild type. Plants were grown for 4 d on Murashige and Skoog/2 + 1% (w/v) Suc. Subsequently, they were exposed to 100 nL L–1 ethylene for 6 d.
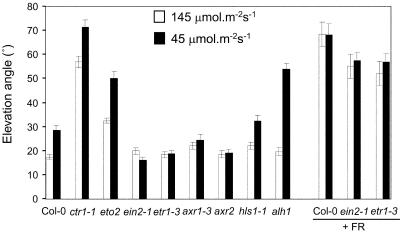
We further investigated the role of ethylene and auxin on leaf angle, induced by low light intensity. Elevation angle was defined by Herbert (1983) as the angle between the leaf midrib and the horizontal. In this study, the elevation angle of the petiole of leaf 6, before full expansion, was used as a parameter to measure hyponastic responses in Arabidopsis mutants (Fig. 3A). When grown under low light intensity, leaves of wild-type plants displayed a more vertical orientation. Ethylene-insensitive mutants etr1-3 and ein2-1 (Guzmán and Ecker, 1990; Chang et al., 1993) did not show any increase in elevation angles in low light intensity and, thus, had flatter rosettes than wild type, although they reacted strongly to low R to FR ratios as did the wild type in these conditions (Fig. 3). This confirms the suggested separation of R to FR effects and light intensity effects in this kind of response (Hangarter, 1997). In contrast to ethylene-insensitive mutants, the ethylene overproducer eto2 (Vogel et al., 1998) had larger leaf elevation angles than wild type. In accordance with this observation, ctr1-1 (constitutive triple response 1; Kieber et al., 1993) petioles had large elevation angles under all tested conditions.
Figure 3.
Ethylene and auxin mutants differ from wild type in elevation angle. Plants were grown on soil at a PPFD of 125 μmol m–2 s–1 (white bars) or 45 μmol m–2 s–1 (black bars), with or without supplemented FR. Error bars = se.
Similar to ethylene-insensitive mutants, the effect of light intensities on the elevation angle in auxin mutants was also less pronounced than in the wild type (Fig. 3). Axr1-3 and axr2 and did not respond. Hls1-1, which is disturbed in differential growth for apical hook formation in dark grown seedlings, had a wild type-like response (Lehman et al., 1996). This indicates that HLS1 does not play a major role in differential growth in petioles. In contrast, alh1 had a stronger reaction than wild-type Arabidopsis.
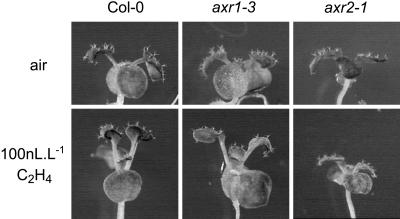
To investigate the relation between ethylene and auxin in this process in more detail, we treated auxin mutants with ethylene for 6 d. The effect was severely attenuated in axr2 and was still very obvious in axr1-3 (Fig. 4). Thus, intact auxin signaling is needed for the ethylene-induced phenotype to occur.
Figure 4.
The auxin-insensitive mutants axr1-3 and axr2-1 are attenuated in the leaf elevation response induced by ethylene. Upper row, Untreated plants; lower row, plants treated with 100 nL L–1 of ethylene for 6 d. Left to right, Col-0, axr1-3, and axr2-1.
Together, these data suggest that low light-induced leaf elevation is dependent of both ethylene and auxin signals and that the ethylene response requires a functional auxin signaling pathway.
Allocation of Leaf Biomass in Ethylene and Auxin Mutants
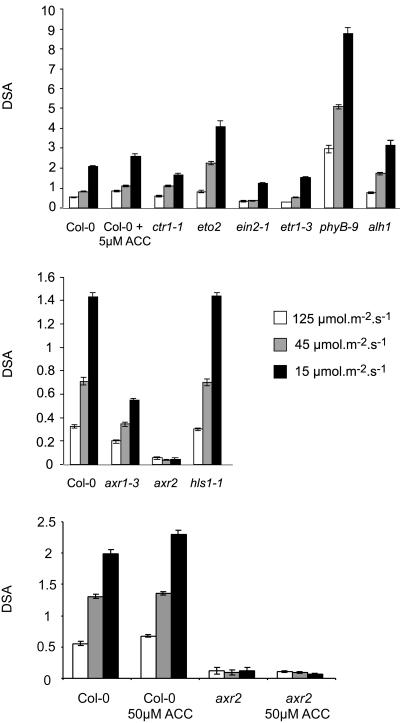
Another characteristic typical of wild-type Arabidopsis plants grown in low R to FR ratios is the increase in elongation of petioles and a reduction of leaf blade surface (Robson et al., 1993). As shown in Figure 5, this was also the case when light intensity was diminished without changing the R to FR ratio. To test whether ethylene and auxin influence the allocation of leaf biomass, we have grown mutants defective in their response to these hormones in different light intensities. Under low light conditions, the ethylene-insensitive mutants (etr1-3 and ein2-1) had larger leaf blades than wild-type plants (Table I). Wild-type plants grown in low light (15 μmol m–2 s–1) had a 30% reduction in leaf blade surface area compared with plants grown in high light intensity (125 μmol m–2 s–1), whereas the blades of ethylene-insensitive mutants even showed a slight increase in size (i.e. 16% for ein2-1; data not shown). Mutants with a constitutive ethylene phenotype, such as eto2 and ctr1-1, and wild-type plants treated with ACC had smaller leaf blades than untreated wild-type plants (Table I). In general, constitutive ethylene responses result in dwarfism (Kieber et al., 1993; Rodrigues-Pousada et al., 1993; Hirayama et al., 1999). Thus, the petiole length is also affected (Table I). However, eto2 was less affected in petiole growth than ctr1-1 or ACC-treated wild type (Fig. 6A; Table I). To correct for the global effect of the mutations on growth, petiole length and leaf blade area have to be taken into account. A parameter that expresses the DSA was defined: DSA = (petiole length)2 × (leaf blade surface)–1. Eto2, an ACS mutant expressing a hyperstable ACS5 protein, is oversensitive to decrease in light intensities, exemplified by higher DSA values than the wild type (Fig. 6; Vogel et al., 1998; Chae et al., 2003). This implies that disturbing ethylene biosynthesis alters the responses to shading. Moreover, light may control ethylene biosynthesis ACS5. In contrast to eto2, ethylene-insensitive mutants had smaller DSA values than wild type (Fig. 6A).
Figure 5.
Ethylene and auxin mutants differ from wild type in allocation of biomass in low light intensities. Plants were grown for 1 week in 45 μmol m–2 s–1 PPFD. Photographs were taken 1.5 weeks after transfer to the indicated light conditions.
Table I.
Leaf surface and petiole length relative to the wild type in specific light conditions
Values are fractions of 1.
| Investigated Trait | Columbia (Col)-0 | 5 μm ACC | 50 μm ACC | ctr1-1 | ein2-1 | eto2 | etr1-3 | phyB-9 | alh1 | axr1-3 | axr2 | hls1-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area 125a | 1.00 | 0.54 | 0.21 | 0.29 | 0.98 | 0.57 | 1.00 | 0.86 | 0.98 | 1.52 | 1.05 | 1.11 |
| Area 15a | 1.00 | 0.64 | 0.19 | 0.24 | 1.63 | 0.59 | 1.79 | 0.65 | 1.19 | 1.09 | 0.94 | 0.97 |
| Petiole length 125a | 1.00 | 0.92 | 0.52 | 0.58 | 0.71 | 0.90 | 0.66 | 2.18 | 1.22 | 1.18 | 0.32 | 1.02 |
| Petiole length 15a | 1.00 | 0.89 | 0.48 | 0.50 | 0.95 | 1.25 | 1.20 | 1.64 | 1.54 | 0.38 | 0.03 | 1.00 |
a No. indicates light intensity (in μmol m-2 s-1).
Figure 6.
Comparison of biomass allocation in ethylene mutants (A), auxin mutants (B), and the effect of exogenous ACC (C), using a defined parameter: degree of similarity to shade avoidance (DSA). DSA = (petiole length)2 × (leaf blade surface area)–1. Plants were grown for 1 week in 45 μmol m–2 s–1 PPFD. After transfer (1.5 weeks) to 125 μmol m–2 s–1 (white bars), 45 μmol m–2 s–1 (gray bars), or 15 μmol m–2 s–1 (black bars), leaf 4 was analyzed for leaf blade surface and petiole length. Error bars = se.
The auxin-insensitive mutants (axr2 and axr1-3) were also impaired in the redistribution of biomass (Fig. 6B). In comparison with wild type, they had short petioles, especially in low light (Table I; Fig. 5). Axr2 had the most severe phenotype. In this mutant, no change was observed. This confirms the necessity for a normal auxin response in the reaction to shading. Continuous supply of 50 μm ACC could not revert the phenotype of the auxin-insensitive mutant axr2 (Fig. 6C).
Regulation of Transcription by Different Light Intensities
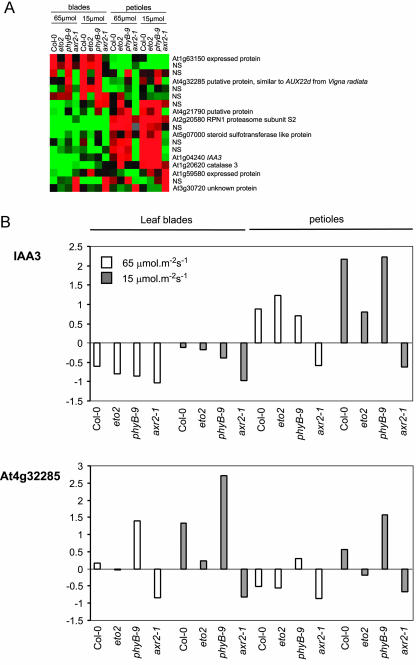
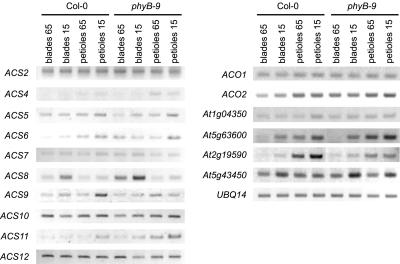
To investigate to what degree changes in gene expression are involved in the above-described responses, we used a modified cDNA-AFLP method to analyze part of the transcriptome (Breyne et al., 2002). Of 800 tags, we isolated 18 fragments of genes that were differentially regulated by low light intensities in wild type but not in the axr2 mutant (Fig. 7A). Five tags were homologous to genes with a known function, whereas four others were not. Nine tags could not be sequenced. Among those that were sequenced, we found CAT3 (catalase 3), a gene known to be negatively regulated by light (McClung, 1997). We also found a steroid sulfotransferase-like gene and RPN1 (regulatory subunit of the 26S proteasome) clustering with CAT3, although RPN1 did not appear to be strongly light regulated. Further, we detected two AUX/IAA-related genes. IAA3 was predominantly expressed in petioles, whereas transcription in leaf blades of a gene with similarity to AUX22d of mung bean (Vigna radiata) was similar to that in petioles. In eto2, its expression was lower than in wild type. Subsequently, we investigated auxin induced ethylene biosynthesis in low light intensities. To that end, we used a reverse transcriptase (RT)-PCR approach for the analysis of ethylene biosynthesis genes. A number of ACSs are known to be auxin induced. Among them are, in ascending order of inducibility, ACS6, ACS4, and ACS8 (Abel et al., 1995; Tian et al., 2002). Steady-state messenger levels of ACS6, ACS8, ACS9, and ACS11 genes were clearly higher in low light intensities (Fig. 8). Other ACSs, ACS2, ACS7, ACS10, and ACS12, were expressed more constitutively. Moreover, in phyB-9 leaf blades, the expression level of ACS6, ACS8, and ACS11 was higher than in wild type in high light intensities. A similar feature was found for ACS4 and ACS9 in phyB-9 petioles. This suggests that these genes may be involved in the constitutive shading phenotype in the absence of active phytochrome. ACS5 also had a higher expression in low light intensities and appeared more petiole specific. This confirms earlier observations of significantly higher ethylene production in dark-grown compared with light-grown eto2 mutants, which have a hyperactive ACS5 (Vogel et al., 1998). The most obvious way to explain the exaggerated shading response of eto2 might at least partly be in this low light (or dark)-specific accumulation of ACS5 combined with additional stabilization of the protein (Chae et al., 2003). As a consequence, a higher ACC level is available for the ACOs in shaded eto2 plants.
Figure 7.
A, Cluster of shade-regulated fragments derived from cDNA-AFLP transcript profiling analysis. Colored squares, Relative transcript expression values of the genes in leaf blades and petioles of rosettes that were grown either in 65 or 15 μmol m–2 s–1 PPFD. Red, Up-regulated; green, down-regulated; black, neutral; gray, missing value. B, Histograms of the expression pattern in leaf blades and petioles of auxin-related genes IAA3 and At4g32285 derived from the semiquantitative analysis of cDNA-AFLP fragments, represented in A. Expression of the genes at a higher light intensity (65 μmol m–2 s–1, white bars) and a lower light intensity (15 μmol m–2 s–1, gray bars) are compared. Values are normalized and express the induction or repression compared with the mean of all samples, which is set as 0. The following formula was used: normalized value = (sample value – mean value of all samples) × (sd of all samples)–1. A value lower than 0 represents a repression and higher than 0 an induction relative to the mean value of all tested samples.
Figure 8.
RT-PCR analysis of ethylene biosynthesis enzymes in wild type and phyB-9 mutants. RNA was prepared from leave blades and petioles from plants grown in 65 μmol m–2 s–1 (65) or 15 μmol m–2 s–1 (15).
To assess the effect on the final step in ethylene biosynthesis, the expression of six ACO genes was studied. Transcript regulation was similar to that of the ACS genes. A number of ACO genes, with At1g5010 (ACO1), At1g62380 (ACO2), and At1g04350 among them, were rather constitutively expressed. In contrast, the putative ACOs, At2g19590 and At5g63600, were clearly induced in leaf blades in low light intensities. This was also the case for At5g43450, albeit to a lesser extent. ACO mRNA levels were never down-regulated in low light intensity.
DISCUSSION
Quality versus Quantity Shading. Can They Be Uncoupled?
Plants react to shading of canopies by detecting changes in light quality, i.e. R to FR ratio (Smith and Whitelam, 1997). Recently, it has become clear that diminishing this ratio triggers a number of auxin transport-dependent responses, including hypocotyl elongation (Steindler et al., 1999). Other studies also have linked photomorphogenesis to auxin responses (for review, see Swarup et al., 2002). We studied the effects of light quantity and found that auxin and ethylene-insensitive mutants have a reduced response to low light intensities. In addition, the induction of auxin up-regulated genes in plants in lower light intensities indicates that phenotypic adaptations upon quantity shading are auxin mediated. One of those genes was IAA3/SHY2. This gene was already shown to be necessary for elongation growth in PHYB-deficient plants (Tian et al., 1999). The role for IAA3/SHY2 in elongation matches its lower expression in wild-type plants in white light compared with dark (Tian et al., 2002). Iaa3/shy2 mutants have a reduction of expression of the IAA7/AXR2 gene (Tian et al., 2002). The reverse relation is also true. Iaa7/axr2 mutants are defective in the expression of IAA3/SHY2 (Fig. 6; Tian et al., 2002). Tian et al. (2002) have suggested that IAA7/AXR2 is positively controlled by IAA3/SHY2 in shoots. Our data suggest a crucial role for both genes in shading. The mutations in iaa7/shy2 and iaa3/axr2 cause defects in auxin-induced hypocotyl elongation (Kim et al., 1996; Vandenbussche et al., 2003). Because they are dominant gain-of-function mutations, a functional redundancy of other IAA genes cannot be ruled out. Nevertheless, IAA3/SHY2 and IAA7/AXR2 remain good indicators for a role for auxin in the elongated shoot phenotype during shading.
At4g32285 is a homolog of the auxin inducible AUX22 genes of mung bean hypocotyls (Yamamoto et al., 1992). The first AUX22 gene was isolated from dark-grown elongating hypocotyl tissues of soybean (Glycine max; Ainley et al., 1988). This again corroborates the role of auxin in the response to diminished light intensities.
Moreover, auxin-inducible ethylene biosynthesis genes had a higher transcript level in phyB-9 mutants (Fig. 7). These plants have a constitutive shade avoidance phenotype, typical for plants grown under low R to FR ratios. Therefore, it is likely that the responses to quantity shading have a similar underlying auxin-dependent mechanism as those upon growth in quality shading, i.e. low R to FR ratios. However, as to the ethylene production rates, quantity and quality shading can be uncoupled. Upon FR enrichment without altering PPFD levels, the ethylene signal is of minor importance because ethylene-insensitive mutants do react to the addition of FR. Moreover, we could not detect any increase in ethylene production. In contrast, lowering light intensity coincided with increased ethylene production, and ethylene-insensitive mutants have a defect in leaf hyponasty.
Biological Significance for Ethylene Biosynthesis in Shading Responses
In Arabidopsis, elevation of leaves has been described as a function of gravity and is influenced by R to FR ratio and light intensity (Fig. 3; Hangarter, 1997). In many species, ethylene can promote negative gravitropism in stems (Wheeler et al., 1986). In Arabidopsis, ethylene stimulates leaves to grow in a vertical position (Fig. 2). This is also an effective mechanism to reach out for light. Prolonged treatments during leaf development were needed to retain the more vertical position. Therefore, it is unlikely that this response is based on the same mechanisms found in the hyponastic response of R. palustris petioles, which is a much faster response, occurring within less than 1 h (Cox et al., 2003).
One of the best known ethylene responses is the inhibition of cell expansion and consequent dwarfism. This has been shown for roots, dark-grown hypocotyls, and light-grown mature plants. Previous work indicated that as leaves expand, ACS1 mRNA levels decrease (Rodrigues-Pousada et al., 1993; renamed ACS2 in The Arabidopsis Information Resource database). We found that ACS6 and ACS8 were up-regulated in shaded plants and that their expression was the highest in leaf blades. These genes are also strongly induced by auxins (Tian et al., 2002). Gene expression of ACSs is regulated by a number of different factors, and, in most cases, a subset is induced by auxins (Yi et al., 1999).
The respective partial and complete failure of ethylene- and auxin-insensitive mutants to react to shading suggests that both hormones may be involved in the same cascade. However, auxin-insensitive mutants could not be rescued by continuously applying exogenous ACC or ethylene. This may not reflect the natural conditions, in which diurnal fluctuations of ethylene production can occur (F. Vandenbussche and D. Van Der Straeten, unpublished data). Thus, timing of the signals may be important. Nonetheless, it is also possible that light intensity exerts its regulation of ethylene synthesis independent of the auxin signal.
Transcription of putative ACO genes, although shade induced, appeared independent of phytochrome B signaling with the exception of At2g19590, which had a lower expression level in phyB-9. However, it remains to be determined whether At2g19590 is a true ACO. Other factors probably exert an effect on expression of ACOs upon shading. One possibility is regulation by other stable phytochromes (Clack et al., 1994). In addition, other photoreceptors, such as cryptochromes or chlorophyll, may mediate the signaling pathway that leads to their transcription.
Apart from regulation of ethylene biosynthesis genes on the transcriptional level, light also has an influence on ethylene production by the modification of ACO. The fast decrease in ethylene production, which we measured during the change from low to high light intensities, might indicate a rapid change of enzyme activity due to a depletion of catalytic CO2 caused by an increase in photosynthetic activity. This confirms previous reports in which light was found to have an inhibitory effect on ethylene biosynthesis in green tissues (Yang and Hoffman, 1984).
PhyB mutants have a higher ethylene production (Fig. 1A; Finlayson et al., 1998). It is known that the amount of chlorophyll in these plants is lower (Reed et al., 1993). Previous work indicated that the effect of light on ethylene production was coupled to the photosynthetic electron transport (De Laat et al., 1981). It also was shown that ethylene decreases electron transport but not Rubisco activity (Wullschleger et al., 1992). Thus, an increase in ethylene production under low light might be due to insufficient capacity to deplete CO2 from the cellular environment, which would leave ACO in a more active state. However, a second possibility is that the effect on ethylene production is directly connected to Rubisco because it was shown in Arabidopsis that RBCS genes and Rubisco activity are up-regulated in higher light intensities (Dedonder et al., 1993; Zhang et al., 2002). Thus, there could be a competition for CO2 among ACOs and Rubisco, with the activity of one enzyme being balanced by the other. However, the extent of control at the level of ACC remains to be investigated because the predominant regulation of ethylene production resides at the level of ACC synthesis, which is a function of protein stability of ACSs (Chae et al., 2003). Nonetheless, it can be concluded that the light control over ethylene production is exerted at different levels, ranging from transcriptional regulation to posttranslational modification and regulated proteolysis.
The reaction of Arabidopsis plants to shading, caused by spectral changes (i.e. FR enrichment) or low light intensity, probably relies to some extent on the same mechanism. This involves a precise control of auxin and ethylene signals and determines the architecture of the leaves.
MATERIALS AND METHODS
Plant Material and Biometrics
Col-0, phyB-9, axr1-3, axr2-1, hls1-1, eto2, ein2-1, and etr1-3 seeds were obtained from the Arabidopsis Biological Resource Center (Columbus, OH). All mutants are of the Col-0 background. Seeds were sown and plants were grown under sterile conditions as described (Smalle et al., 1997). The plants were subjected to a light intensity of 65 μmol m–2 s–1 for 1 week and then transferred to light conditions as indicated. Variety in light intensities was obtained by adding or removing cool-white light tubes or by shading with layers of Miracloth (Calbiochem Biosciences, La Jolla, CA), which did not alter the light quality, as assessed by measuring the spectrum with a portable spectroradiometer, Li-1800 (LI-COR, Lincoln, NE).
For the leaf biomass distribution experiments, plants were grown on a medium containing one-half-strength Murashige and Skoog salts and 1% (w/v) Suc in cool-white light (Lumilux Plus, Osram, Germany) under a photoperiod of 16 h of light/8 h of dark at 22°C. Three days after the emergence of leaves 5 and 6, corresponding to approximately 2.5 weeks at midday, leaves 3 and 4 were harvested and stuck to paper with tape. An image was acquired using a flatbed scanner. Analysis of the petiole length and leaf blade surfaces was done with ScionImage software (Scion Corp., Frederick, MD).
For the study of leaf angles, plants were grown on soil in cool-white light (Lumilux Plus) under the same photoperiod as above. When the fourth leaf pair emerged, plants were taken from the soil at midday and meticulously dissected such that only the hypocotyl, the apical meristem, the petiole, and the midvein of leaf 6 were left. These were very carefully stuck onto paper without disturbing the original inclination. An image was acquired using a flatbed scanner. Analysis of the elevation angles (this is the angle between petiole and the horizontal) was done with ScionImage software (Scion Corp.).
Gas Measurements
Plants were grown on Murashige and Skoog/2 medium containing 1% (w/v) Suc and 50 μm ACC in 16 h of light/8 h of dark at 22°C and 60% relative humidity. To compare the effect of low light and higher light intensities on ethylene biosynthesis, 2-week-old Col-0 and phyB-9 plants were grown in 120 or 30 μmol m–2 s–1 PPFD, respectively, for 3 d to minimize effects of circadian rhythms. After that, 12 plants were put in air-tight 100-mL glass vials while remaining on the same medium. Ethylene production of wild-type Arabidopsis plants was too low for continuous flow measurements. Therefore, we measured ethylene after accumulation in a closed vial. Every 2 h, the vials were flushed at a flow rate of 1 L h–1, and ethylene was measured.
For the light switch experiment, plants were essentially in the same conditions as mentioned above. Variety in light intensity was achieved by adding or removing cool-white light tubes. Changes in R to FR ratio were obtained by adding filtered incandescent light from a 60-W bulb. The R to FR and PPFD values were determined using an Li-1800 (LI-COR) portable spectroradiometer. After 2 weeks, they were put in glass jars for gas measurements while still on the same medium. The jars were fit into a continuous flow system, connected to a photo-acoustic detector for measuring ethylene (Bijnen et al., 1996). CO2 was measured using a URAS 14 apparatus (Hartmann & Braun, Frankfurt, Germany).
Transcript Analysis
Leaf blades and petioles of 2.5-week-old plants, grown on Murashige and Skoog/2 + 1% (w/v) Suc in 16 h of light/8 h of dark at 22°C, were harvested at midday and frozen in liquid nitrogen. RNA was prepared using QIAGEN RNeasy (QIAGEN GmbH, Hilden, Germany). RNA was treated with Dnase amplification grade (Life Technologies/Gibco-BRL, Cleveland).
To get an overview of gene expression, we used a modified cDNA-AFLP technique (Breyne et al., 2002). Twenty primer combinations were tested. A total of 800 fragments were analyzed corresponding to 4% to 5% of the total genome. Cluster analysis was performed using the Eisen software (http://rana.lbl.gov/EisenSoftware.htm) performing hierarchical clustering. Two biological repeats were done. Fragments that had a cutoff value (sd on mean/mean of all samples) smaller than 0.3 or that were not repeatable in clustering were discarded. The remaining fragments that fell into a cluster of interest were sequenced. Only fragments that produced a sequence with an “expect value” of less than 10–5 in a BLAST search were retained.
RT-PCR with gene-specific primers was also performed. For ACS4, ACS6, ACS8, all ACO genes, and UBQ 14, the pre-amplification reactions of the cDNA-AFLP were used as template material for the gene-specific PCR. For all other genes, the gene-specific PCRs were done directly on cDNA that was obtained by a classical RT reaction according to protocol (Invitrogen, Carlsbad, CA). All PCRs were done in a Mastercycler (Eppendorf, Hamburg, Germany). Cycles had 30 sec at 95°C, 35 sec at hybridization temperature, and 30“at 72°C. A list of gene-specific primers and reaction conditions is given in Table II. Separation was done on a 1% (w/v) agarose gel. DNA was stained with ethidium bromide in the gel.
Table II.
RT-PCR primers, cycle no., and hybridization temperatures
| Gene | Primer Set | No. of Cycles | Hybridization Temperature |
|---|---|---|---|
| ACS2 (At1g01480) | 5′AGATCGTCGAGAAAGCATCTG3′ | 30 | 56°C |
| 5′GAAGAGGTGAGTGTGGTGAC3′ | |||
| ACS4 | 5′GTTTACGAAGTGAAGCTCAAC3′ | 35 | 56°C |
| 5′GTCTCATCAATCATGTTCGCG3′ | |||
| ACS5 | 5′GCGGCAAGTCTCAAGAGGA3′ | 28 | 54°C |
| 5′TTCTGGGCTTGTTGGTAAGC3′ | |||
| ACS6 | 5′CTGAATCTATTGTCTAAAATCGC3′ | 30 | 55°C |
| 5′ACGCATCAAATCTCCACAAAG3′ | |||
| ACS7 | 5′TATTTTGTTGGATGAATTTGGGT3′ | 30 | 56°C |
| 5′TTCTCTTCAACGCAATCTCC3′ | |||
| ACS8 | 5′GTCCAGTTTCGGTCTAATCTC3′ | 28 | 55°C |
| 5′ATAGGTGTCTCATGTCAACCC3′ | |||
| ACS9 | 5′TCGGTTTACCAGGTTTTCGC3′ | 30 | 55°C |
| 5′ACACGAGTTTCTTCTGACGAA3′ | |||
| ACS10 | 5′ACAGGCAGAGATTGCAGAG3′ | 30 | 55°C |
| 5′ACTGAAACAGATACGGAACC3′ | |||
| ACS11 | 5′CAGTTGTTTGAAGAGTAACGC3′ | 30 | 55°C |
| 5′TAACAGGAAAGCTTGGAGA3′ | |||
| ACS12 | 5′AGAGCTGGAGTCATCTACTCC3′ | 30 | 55°C |
| 5′GCAAGCTGTCTGATTCGTTCC3′ | |||
| ACO (At5g63600) | 5′CCTGTCTACTGAAAACCCTC3′ | 30 | 56°C |
| 5′GTCTCCTTGAACAATTCATCA3′ | |||
| ACO (At1g04350) | 5′GCATTCACTAAAATTATACA3′ | 28 | 56°C |
| 5′CAAATAAGTAAACCATTTCCT3′ | |||
| ACO (At1g05010) | 5′GATCTGCTGTGCGAGAATCTC3′ | 28 | 56°C |
| 5′TAAATAACCCTTCTCTAAACC3′ | |||
| ACO (At1g62380) | 5′CCAGCTACTTCGCTTGTCGAG3′ | 27 | 56°C |
| 5′GTCTCTACGGCTGCTGTAGGA3′ | |||
| ACO (At2g19590) | 5′CAGAGGAACTCAGCAAGACG3′ | 28 | 56°C |
| 5′ATCCGTATGTTCTCTCAGCC3′ | |||
| ACO (At5g43450) | 5′GTACAAAGATATCACCATACCAG3′ | 28 | 56°C |
| 5′TGGTTGAGGAACTCTATAGC3′ | |||
| UBQ14 | 5′GATCCAGGACAAGGAAGGTATC3′ | 25 | 55°C |
| 5′AGCCTTAGCACCAAGTGAAGG3′ |
Acknowledgments
The authors wish to thank Mira Haegman for technical assistance and the sequencing group at the Plant Systems Biology lab of Ghent university for all their good work.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.022665.
This work was supported by the Fund for Scientific Research (Flanders; grants no. G.0281.98, WO.004.99, and G.0345.02 to D.V.D.S.), by the Flanders Interuniversity Institute of Biotechnology, and by the European Union (grant nos. EU–RTN–INTEGA and HPRN–CT–2000–00090).
References
- Abel S, Nguyen MD, Chow W, Theologis A (1995) ACS4, a primary indole acetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. J Biol Chem 270: 26020–26020 [DOI] [PubMed] [Google Scholar]
- Ainley WM, Walker JC, Nagao RT, Key JL (1988) Sequence and characterization of 2 auxin-regulated genes from soybean. J Biol Chem 63: 10658–10666 [PubMed] [Google Scholar]
- Al Shehbaz IA, O'Kane SL (2002) Taxonomy and phylogeny of Arabidopsis (Brassicaceae). In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0001, http://www.aspb.org/publications/arabidopsis [DOI] [PMC free article] [PubMed]
- Bassi PK, Spencer MS (1983) Does light inhibit ethylene production in leaves? Plant Physiol 73: 758–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnen FGC, Reuss J, Harren FJM (1996) Geometrical optimization of a longitudinal resonant photoacoustic cell for sensitive and fast trace gas detection. Rev Sci Instrum 67: 2914–2923 [Google Scholar]
- Blackman GE, Wilson GL (1951) Physiological and ecological studies in the analysis of plant environment: VII. An analysis of the differential effects of light intensity on the net assimilation rate, leaf-area ratio, and relative growth rate of different species. Ann Bot 15: 373–408 [Google Scholar]
- Breyne P, Dreesen R, Vandepoele K, De Veylder L, Van Breusegem F, Callewaert L, Rombauts S, Raes J, Cannoot B, Engler G et al. (2002) Transcriptome analysis during cell division in plants. Proc Natl Acad Sci USA 99: 14825–14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock TG, Ghosheh NS, Kaufman PB (1994) Differential sensitivity to indole-3-acetic acid and gibberellic acid following gravistimulation of the leaf sheath pulvini of oat and barley. Plant Physiol Biochem 32: 487–491 [Google Scholar]
- Chae HS, Faure F, Kieber JJ (2003) The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15: 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene response gene ETR1: Similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chapin FS, Walker LR, Fastie CL, Sharman LC (1994) Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecol Monogr 64: 149–175 [Google Scholar]
- Chatfield SP, Stirnberg P, Forde BG, Leyser O (2000) The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 24: 159–169 [DOI] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA (1994) The phytochrome apoprotein family in Arabidopsis is encoded by 5 genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol 25: 413–427 [DOI] [PubMed] [Google Scholar]
- Clua A, Bottini R, Brocchi GN, Bogino J, Luna V, Montaldi ER (1996) Growth habit of Lotus tenuis shoots and the influence of photosynthetic photon flux density, sucrose and endogenous levels of gibberellins A-1 and A-3. Physiol Plant 98: 381–388 [Google Scholar]
- Colon-Carmona A, Chen DL, Yeh KC, Abel S (2000) AUX/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol 124: 1728–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbineau F, Rudnicki RM, Goszczynska DM, Come D (1995) The effect of light quality on ethylene production in leaves of oat seedlings (Avena sativa L.). Environ Exp Bot 35: 227–233 [Google Scholar]
- Cox MCH, Millenaar FF, de Jong van Berkel YEM, Peeters AJM, Voesenek LACJ (2003) Plant movement: submergence-induced petiole elongation in Rumex palustris depends on hyponastic growth. Plant Physiol 132: 282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedonder A, Rethy R, Fredericq H, Van Montagu M, Krebbers E (1993) Arabidopsis RBCS genes are differentially regulated by light. Plant Physiol 101: 801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laat AMM, Brandenburg DCC, van Loon LC (1981) The modulation of the conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene by light. Planta 153: 193–200 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Halliday KJ, Harberd NP, Whitelam GC (1996) The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: novel phytochromes control internode elongation and flowering time. Plant J 10: 1127–1134 [DOI] [PubMed] [Google Scholar]
- Finlayson SA, Lee IJ, Morgan PW (1998) Phytochrome B and the regulation of circadian ethylene production in sorghum. Plant Physiol 116: 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson SA, Lee IJ, Mullet JE, Morgan PW (1999) The mechanism of rhythmic ethylene production in sorghum: the role of phytochrome B and simulated shading. Plant Physiol 119: 1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124: 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangarter RP (1997) Gravity, light and plant form. Plant Cell Environ 20: 796–800 [DOI] [PubMed] [Google Scholar]
- Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12: 757–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert TJ (1983) The influence of axial rotation upon interception of direct solar radiation by plant leaves. J Theor Biol 105: 603–618 [Google Scholar]
- Hirayama T, Kieber JJ, Hirayama N, Kogan M, Guzman P, Nourizadeh S, Alonso JM, Dailey WP, Dancis A, Ecker JR (1999) Responsive-to-antagonist1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell 97: 383–393 [DOI] [PubMed] [Google Scholar]
- Holmes MG (1983) Perception of shade. Philos Trans R Soc Lond B 303: 503–521 [Google Scholar]
- Kao CH, Yang SF (1982) Light inhibition of the conversion of 1-aminocyclopropane carboxylic acid to ethylene in leaves is mediated through carbon dioxide. Planta 155: 261–266 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Kim BC, Soh MS, Kang BJ, Furuya M, Nam HG (1996) Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor mutations of hy2. Plant J 9: 441–456 [DOI] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85: 183–194 [DOI] [PubMed] [Google Scholar]
- Leyser O (2002) Molecular genetics of auxin signaling. Annu Rev Plant Biol 53: 377–398 [DOI] [PubMed] [Google Scholar]
- Lippincott BB, Lippincott JA (1971) Auxin-induced hyponasty of the leaf blade of Phaseolus vulgaris. Am J Bot 58: 817–826 [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP (1980) Genetic-control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L) Heynh. Z Pflanzenphysiol 100: 147–160 [Google Scholar]
- Madlung A, Behringer FJ, Lomax TL (1999) Ethylene plays multiple non-primary roles in modulating the gravitropic response in tomato. Plant Physiol 120: 897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliakal SK, McDonnell K, Dudley SA, Schmitt J (1999) Effects of red to far-red ratio and plant density on biomass allocation and gas exchange in Impatiens capensis. Int J Plant Sci 160: 723–733 [Google Scholar]
- McClung CR (1997) Regulation of catalases in Arabidopsis. Free Radic Biol Med 23: 489–496 [DOI] [PubMed] [Google Scholar]
- McConnaughay KDM, Coleman JS (1999) Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology 80: 2581–2593 [Google Scholar]
- Müller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Darry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M, Schmitt J (1999) Genes affecting phenotypic plasticity in Arabidopsis: pleiotropic effects and reproductive fitness of photomorphogenic mutants. Evol Biol 12: 551–562 [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PRH, Whitelam GC, Smith H (1993) Selected Components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome. Plant Physiol 102: 1179–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Pousada RA, De Rycke R, Dedonder A, Van Caeneghem W, Engler G, Van Montagu M, Van Der Straeten D (1993) The Arabidopsis 1-aminocyclopropane-1-carboxylate synthase gene 1 is expressed during early development. Plant Cell 5: 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki RM, Fjeld T, Moe R (1993) Effect of light quality on ethylene formation in leaf and petal disks of Begonia × hiemalis-Fotsch cv Schwabenland red. Plant Growth Regul 13: 281–286 [Google Scholar]
- Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, Yoshida S, Koshiba T (2002) The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J 32: 1011–1022 [DOI] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van Der Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H (1992) The ecological functions of the phytochrome family: clues to a transgenic programme of crop improvement. Photochem Photobiol 56: 815–822 [Google Scholar]
- Smith H, Whitelam GC (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20: 840–844 [Google Scholar]
- Somers DE, Sharrock RA, Tepperman JM, Quail PH (1991) The Hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome-B. Plant Cell 3: 1263–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I (1999) Shade avoidance responses are mediated by the ATHB-2 HD-Zip protein, a negative regulator of gene expression. Development 126: 4235–4245 [DOI] [PubMed] [Google Scholar]
- Swarup R, Parry G, Graham N, Allen T, Bennett M (2002) Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol 49: 411–426 [DOI] [PubMed] [Google Scholar]
- Tian Q, Reed JW (1999) Control of auxin-regulated root development by the Arabidopsis thaliana SH Y2/IAA3 gene. Development 126: 711–721 [DOI] [PubMed] [Google Scholar]
- Tian Q, Reed JW (2001) Molecular links between light and auxin signaling pathways. J Plant Growth Regul 20: 274–280 [Google Scholar]
- Tian Q, Uhlir NJ, Reed JW (2002) Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 14: 301–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin VM, Bradford KJ (1989) Auxin and ethylene regulation of petiole epinasty in 2 developmental mutants of tomato, diageotropica and epinastic. Plant Physiol 90: 1341–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Smalle J, Le J, Madeira-Saibo NJ, De Paepe A, Chaerle L, Tietz O, Smets R, Laarhoven LJJ, Harren FJM et al. (2003) The Arabidopsis thaliana mutant alh1 illustrates a cross-talk between ethylene and auxin. Plant Physiol 131: 1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ (1998) Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA 95: 4766–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watahiki M-K, Yamamoto K-T (1997) The massugu1 mutation of Arabidopsis identified with failure of auxin-induced growth curvature of hypocotyl confers auxin insensitivity to hypocotyl and leaf. Plant Physiol 115: 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RM, White RG, Salisbury FB (1986) Gravitropsim in higher plant shoots: IV. Further studies on participation of ethylene Plant Physiol 82: 534–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger SD, Hanson PJ, Gunderson CA (1992) Assessing the influence of exogenous ethylene on electron-transport and fluorescence quenching in leaves of Glycine max. Environ Exp Bot 32: 449–455 [Google Scholar]
- Yamamoto KT, Mori H, Imaseki H (1992) CDNA cloning of indole-3-acetic acid-regulated genes: AUX22 and SAUR from mung bean (Vigna radiata) hypocotyl tissue. Plant Cell Physiol 33: 93–97 [Google Scholar]
- Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol 35: 155–189 [Google Scholar]
- Yi HC, Joo S, Nam KH, Lee JS, Kang BG, Kim WT (1999) Auxin and brassinosteroid differentially regulate the expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in mung bean (Vigna radiata L.). Plant Mol Biol 41: 443–454 [DOI] [PubMed] [Google Scholar]
- Zhang N, Kallis RP, Ewy RG, Portis AR (2002) Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc Natl Acad Sci USA 99: 3330–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]