Abstract
Tomato (Lycopersicon esculentum var. Better Boy) plants were transformed with a tomato leaf wound-inducible polygalacturonase (PG) β-subunit gene in the antisense orientation (PGβS-AS) under the control of the cauliflower mosaic virus 35S promoter. The leaves of the transgenic plants exhibited small localized lesions, which eventually enlarged and spread throughout the entire surfaces of the leaves, resulting in cell death. The same lesions were also observed in the peduncle of developing flowers, extending to the whole flower causing abscission, resulting in a sterile phenotype. Leaves of transgenic plants exhibited elevated levels of PG activity, hydrogen peroxide, and enhanced defense signaling in response to wounding and elicitor treatment. The defense signaling increased was accompanied by an increased resistance toward tobacco hornworm (Manduca sexta) larvae. The cumulative results suggest that in the absence of the β-subunit protein in tomato leaves, an increase in PG activity occurred that led to an enhanced wound response, the formation of lesions leading to severe necrosis, and an abscission of developing flowers.
The controlled degradation of plant cell wall matrices by polygalacturonases (PGs) can initiate various physiological processes in plants, including fruit ripening (Handfiel and Bennett, 1998), leaf and flower abscission (Kalaitzis et al., 1997), pod dehiscence (Petersen et al., 1996), pollen maturation (Pressey, 1991), cell expansion (Sitrit et al., 1999), nitrogen fixation (Muñoz et al., 1998), and defense gene activation (Ryan and Farmer, 1991; Albersheim et al., 1992). In tomato (Lycopersicon esculentum) fruit, where PG enzymes have been studied in most detail, the catalytic PG (PGcat) is transcriptionally activated at the onset of ripening (Grierson et al., 1987; DellaPenna et al., 1996; Handfiel and Bennett, 1998), and both the mRNA and protein accumulate to high levels (Brady et al., 1982; DellaPenna et al., 1987, 1989). The enzyme is tightly associated with ancillary glycoproteins that are collectively called β-subunits (Mosrefi and Luh, 1983; Knight et al., 1988; DellaPenna et al., 1996). The β-subunit proteins have molecular weights of 37 to 39 kD and are comprised almost entirely of tandem 14-amino acid repeats (Zheng et al., 1992; DellaPenna et al., 1996). In ripening fruit, a β-subunit protein can alter the enzyme activity of PGcat, but the mode of interaction with the enzyme is not known. The protein has been proposed to interact with PGcat to inhibit its catalytic activity or to prevent access of the enzyme to cell wall pectins (Zheng et al., 1992; Watson et al., 1994; DellaPenna et al., 1996). The level of PGcat produced in tomato fruit has been associated in vivo with the level of β-subunit protein that is present (Giovannoni et al., 1989; DellaPenna et al., 1990; Pogson et al., 1991).
During an investigation of defense gene expression in leaves of tomato plants in response to wounding and systemin, a PGcat mRNA and a PGcat regulatory β-subunit mRNA, were found to increase (Bergey et al., 1999). The PGcat gene represents a new subfamily of PG, and the wound-inducible β-subunit gene is identical to a β-subunit homolog (Zheng et al., 1992) previously thought to be a pseudo-gene (DellaPenna et al., 1996).
The temporal expression patterns of the tomato leaf PGcat gene and β-subunit gene differ from those found in fruit. In fruit, β-subunit gene expression occurs just preceding the onset of ripening and then ceases, coinciding with an increase in PGcat gene expression (DellaPenna et al., 1996). In leaves, the PGcat gene is expressed within 1 h after wounding and maximizes at about 6 to 9 h (Bergey et al., 1999). The β-subunit gene is weakly expressed in leaves until about 3 to 4 h after wounding, then increases by 9 h to high levels. PG activity does not correspond to the PGcat gene expression but decreases at about 9 h as the PGcat mRNA is maximally expressed, suggesting that the synthesis of the β-subunit is modulating PGcat activity (Bergey et al., 1999).
The identification of wound-inducible PGcat activity in leaves provided evidence for a new interpretation of wound-induced defense signaling events in tomato leaves (Bergey et al., 1999; Orozco-Cárdenas et al., 2001). The signaling pathway now includes a role for wound-inducible (and jasmonic acid [JA]-inducible) PGcat in producing oligogalacturonides (OGAs) from cell walls that act as signals for the induction of late expressed defense genes (Orozco-Cárdenas et al., 2001). OGAs are known elicitors of hydrogen peroxide (H2O2) generation in plants, leading to defense gene activation (Low and Merida, 1996; Lamb and Dixon, 1997; Bolwell, 1999; Orozco-Cárdenas and Ryan, 1999). A set of early wound-inducible signal pathway-associated genes are expressed in response to systemin and JA, whereas a set of late defense-associated genes are activated by H2O2 (Orozco-Cárdenas et al., 2001). Wound-inducible PG activity coincided with the early signaling-associated genes and appears to play a role in releasing pectic fragments that induce H2O2 for late gene expression. The synthesis of the β-subunit appears to play a role in negatively regulating PGcat and late gene expression.
We report herein that transformation of tomato plants with an antisense β-subunit gene results in an increase of PGcat activity resulting in increased levels of H2O2 in the leaves and the potentiation of late defense gene expression. Expression of the antisense β-subunit gene also results in leaf lesion formation leading to cell death and an early flower abscission precluding fruit development. These results support our hypothesis that the expression of the β-subunit gene in tomato leaves plays an important role in regulating PGcat activity in tomato plants that is associated with both defensive and developmentally related processes.
RESULTS
PGβS-AS Plants Exhibit a Necrotic Leaf-Sterile Phenotype
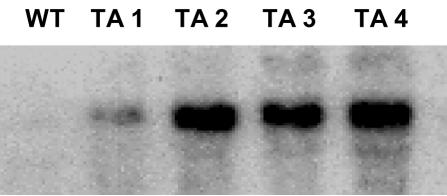
Two groups of 12 plants were transformed with either a vector harboring the wound-inducible PG β-subunit cDNA in the antisense orientation (called the PGβS-AS gene) or an empty vector. The expression of the PGβS-AS gene in leaves of regenerated plants was confirmed by gene-blot analyses using a full-length β-subunit cDNA (Bergey et al., 1999). Figure 1 shows mRNA-blot analyses of a wild-type tomato plant used as a control and four independent transformants (TA1, TA2, TA3, and TA4) that were selected to demonstrate the variability in expression of the antisense gene. The PGβS-AS transformants exhibited an abnormal phenotype (Fig. 2), with lesions forming on the upper surfaces of young expanding leaves near the veins and on the abaxial surface (Fig. 2, C–F). The lesions, with time, covered extensive areas of the leaf blade and led to cell death (Fig. 2E). Lesions were also observed in the peduncle of developing flowers, extending to the whole flower, causing abscission (Fig. 2F). As a consequence, no fruit could be obtained from any of the transgenic antisense plants.
Figure 1.
RNA-blot analysis showing the expression of antisense PG β-subunit mRNA in leaves of wild-type (WT) and transgenic (TA) tomato plants. Twenty micrograms of total leaf RNA was isolated from 6- to 8-week-old tomato leaves, separated by electrophoresis, and transferred to nylon membranes and exposed to film as described in “Materials and Methods.” The PG β-subunit mRNA was identified using a full-length β-subunit cDNA clone. Each lane corresponds to an independently transformed line.
Figure 2.
Phenotype of PGβS-AS transgenic tomato plants. A, Upper surface of a typical wild type tomato leaflet. B, Abaxial surface of the same leaflet. C, Upper surface of a typical leaflet of a tomato plant transformed with the PGβS-AS gene. D, Abaxial surface of the same leaflet. E, Lateral leaflets from a typical leaf of a plant transformed with the PGβS-AS gene. F, Typical inflorescences from wild-type (left) and transgenic (right) plants. No fruit developed on the transgenic plants.
Transgenic PGβS-AS plants were maintained by pruning and clonal propagation (Hu and Wang, 1983; Wang and Arntzen, 1991). To confirm that the symptoms were not pathological in origin, PGβS-AS plants were propagated by standard shoot tip culture methods to obtain pathogen-free plants (Styer and Chin, 1984). Plants that were generated from apical shoot meristems were transferred to soil and grown to full maturity in a controlled environment for 17 h under a light intensity of 300 μE m–2 s– 1 at 28°C and for 7 h in darkness at 18°C. Similar lesions were found in the regenerated PGβS-AS plants. Furthermore, classical attempts to grow and/or isolate known pathogens from the lesions by classical methods were unsuccessful (data not shown).
To facilitate uniformity among comparative analyses performed with transformants and wild-type plants, all the PG activities and H2O2, proteinase inhibitor, and mRNA analyses were performed with symptomatic leaves before the appearance of necrotic lesions (compare with Fig. 2, C and D).
Constitutive Expression of the PGβS-AS Transgene Increases PG Activity in Tomato Leaves
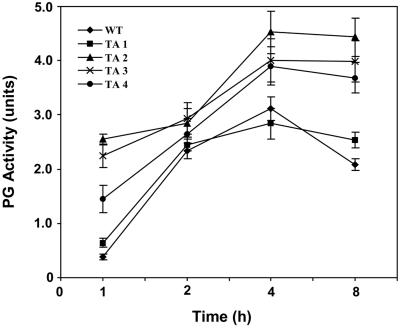
The PG β-subunit has been proposed to play a role in regulating PG activity in tomato fruit (DellaPenna et al., 1996) and in wounded tomato leaves (Bergey et al., 1999). To assess the effects of the constitutive expression of the PGβS-AS gene on PG activity in tomato leaves, PG activity was assayed in leaves of mature plants before and after wounding. Total soluble protein was extracted, partially purified, and assayed for PG activity with time. The basal levels of PG activity found in the leaves of TA2, TA3, and TA4 transgenic plants were much higher than found in the wild-type control leaves or in the TA1 plants (Fig. 3) The PG activity that was induced by wounding in leaves of TA2, TA3, and TA4 were much higher than in leaves of the wild-type or TA1 plants, with maximal activity occurring in all cases at about 4 h (Fig. 3).
Figure 3.
PG enzyme activity in leaves from plants expressing an antisense PG β-subunit gene compared with wild-type plants. PG enzyme assays were performed on leaf extracts from at least two randomly selected, non-necrotic symptomatic leaves from each plant. Values are the average values (±sd) of three measurements per plant. A unit of PG was defined as the amount that caused a 0.1 OD increase at 520 nm per 30 min at 37°C per 0.1 g fresh weight. All assays were repeated three times in duplicate.
PGβS-AS Plants Exhibit Elevated Constitutive and Wound-Inducible Levels of H2O2
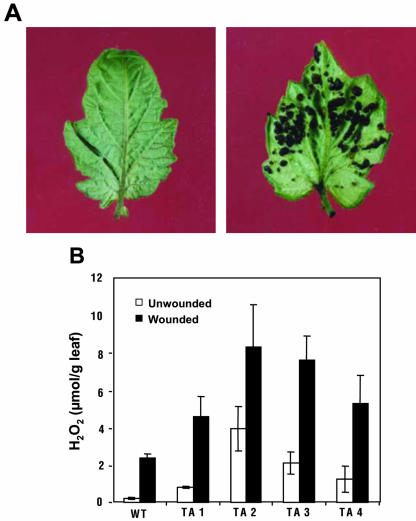
To investigate whether the symptomatic leaves of the antisense plants were accumulating H2O2 corresponding to the increases in PG activity, the 3,3-diaminobenzidine (DAB) colorimetric assay was used to visually detect the presence of H2O2 in the leaves. In excised leaves from wild-type and transgenic antisense plants that had imbibed a 1 mg mL–1 solution of DAB for 1 h, a visual DAB-H2O2 reaction product accumulated only at sites on the transgenic plants where necrotic lesions were developing (Fig. 4A). The DAB stain was heavier in the lesions developing in the abaxial side of the transgenic leaves indicated that the lesions were associated with the accumulation of high levels of H2O2. Because it is known that wounding activates the synthesis of H2O2 in tomato leaves, the levels of H2O2 in both unwounded and wounded leaves of the transgenic plants were measured by means of a quantitative fluorometric assay. The levels are compared with those of similarly treated wild-type plants in Figure 4B. The basal levels of H2O2 in untreated transgenic leaves that have developed necrotic symptoms were from about 1 to 4 μmol g–1 leaf tissue, compared with about 0.2 μmol g–1 tissue in the wild-type controls. Four hours after wounding, the levels of H2O2 in leaves of both wild-type and antisense plants reached maximum levels that were several times higher than in the leaves of wild-type plants (Fig. 4B).
Figure 4.
Accumulation of H2O2 in leaves from wild-type and PGβS-AS transgenic tomato plants. A, Terminal leaflet from a 6- to 8-week-old wild-type plant (left) and from a transgenic plant (right) were excised and vacuum infiltrated with a solution of DAB. After 1 h of stain development, the leaflets were photographed. B, Assays of H2O2 levels in leaf extracts from 6-week-old wild-type plants and from transgenic tomato plants of similar age expressing the PGβS-AS gene. Quantification of H2O2 from extracts was performed as described in “Materials and Methods.” Data are means ± sd; n = 6.
PGβS-AS Plants Exhibit Constitutive Expression of Wound-Inducible Defensive Genes
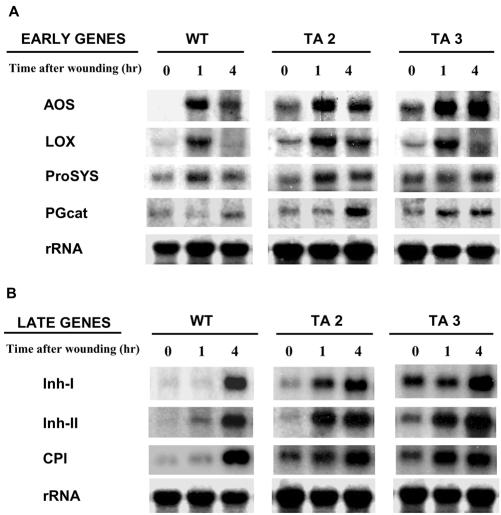
Over 20 wound-inducible defensive genes have been identified in tomato leaves (Bergey et al., 1996). The genes fall into two categories: early expressed genes that are associated with the signaling pathway itself and later expressed genes that are directly related to defense and that have been shown to be associated with an increase in H2O2 in leaves (Orozco-Cárdenas et al., 2001). The possibility that the H2O2 produced by the expression of the PGβS-AS gene might be activating the expression of wound-inducible genes was investigated by gel-blot analyses. Total RNA isolated from young leaves exhibiting small lesions was analyzed for the wound-inducible expression of four of the early genes including allene oxide synthase, lipoxygenase, prosystemin, and PG-cat, and three of the late genes including inhibitor I (Inh I), Inh II, and carboxypeptidase inhibitor (CPI). At time 0, low levels of mRNAs encoding for all the early and late genes could be detected in leaves of unwounded antisense plants but not in the leaves from wild-type plants. The mRNA levels of early genes in both wild-type and antisense plants had typically increased within 1 h after wounding (Fig. 5A). However, the three late genes were strongly expressed in the transgenic plants at 1 h, in contrast to their expression at 4 h in wild-type plants (Fig. 5B). These genes were even more strongly expressed at 4 h in the antisense plants.
Figure 5.
The induction of mRNAs of early and late wound-inducible defense genes in wild-type and PGβS-AS transgenic tomato plants using RNA gel-blot analyses. Total leaf RNA was isolated from leaves, collected at the times indicated, and 20 μg from each time period was separated by electrophoresis, transferred to nylon membranes, and exposed to film as described in “Materials and Methods.” A, Membranes were probed for the induction of early expressed mRNAs using full-length cDNA clones for allene oxide synthase (AOS), lipoxygenase (LOX), Prosystemin (ProSYS), and PG catalytic subunit (PGcat). B, Membranes were probed with full-length cDNAs clones for proteinase Inh I, proteinase Inh II, and CPI. An 18S ribosomal RNA gene probe was used as a loading control.
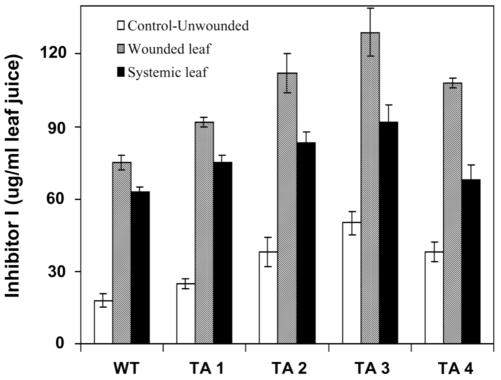
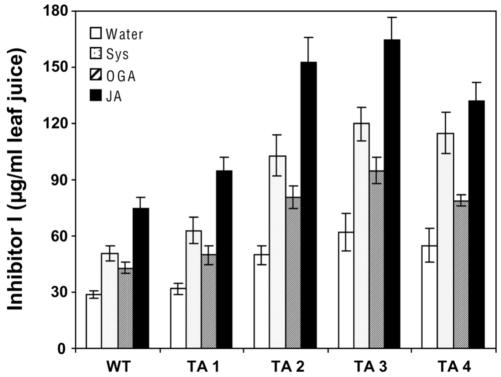
To determine if the apparently large increases in proteinase inhibitor mRNAs in response to wounding were accompanied by similar increases in proteinase inhibitor proteins, the protein levels of one wound-inducible inhibitor, Inh I, was quantified in wounded leaves and upper, unwounded leaves of four transgenic tomato plants. The levels are compared with levels in leaves of wounded control plants in Figure 6. Transgenic plants supplied with systemin, OGA, and JA through their cut stems also induced strikingly higher levels of Inh I protein over levels induced in leaves of wild-type control plants (Fig. 7).
Figure 6.
Quantification of proteinase Inh I in expressed juice from leaves of wild-type and PGβS-AS transgenic tomato plants. Leaf juice was expressed from 6- to 8-week-old tomato plants and assayed for proteinase Inh I content using immunological radial diffusion assays. Data are means ± sd; n = 6.
Figure 7.
Elicitor-enhanced accumulation of Inh I in excised wild-type and PG βS-AS tomato plants. Excised tomato leaves were supplied through their cut petioles with either 0.01 m phosphate buffer (pH 6.0; Control) or with the buffer containing each of the following elicitors for 30 min under light: 25 nm systemin (Sys), 250 μg mL–1 oligo-GalUA (OGA), or 100 μm JA. The leaves were transferred to water and incubated for 24 h under light and then assayed immunologically for Inh I content. Data are means ± sd; n = 6.
PGβS-AS Transgenic Plants Exhibit Increased Resistance toward Tobacco Hornworm (Manduca sexta) Larvae
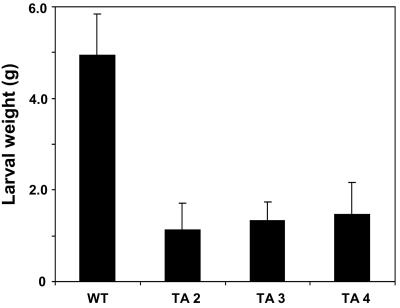
Transgenic tomato plants constitutively expressing high levels of defense-related proteins had been shown previously to be more resistant to insect attacks than wild-type tomato plants (Orozco-Cárdenas et al., 1993; McGurl et al., 1994; Howe et al., 1996; Li et al., 2002). To determine if the PGβS-AS plants that induce high levels of defense gene expression when wounded would have an increased resistance to chewing insects, newly hatched tobacco hornworm larvae were allowed to feed on leaves excised from both PGβS-AS and wild-type plants. The leaves from PGβS-AS plants were young expanding leaves that exhibited localized lesions but had not become necrotic. The leaves were replaced daily with leaves from the same developmental stage with only localized lesions. Figure 8 shows that the weights of larvae fed on leaves from different PGβS-AS transgenic lines are 2 to 3 times smaller than the larvae fed on the wild-type leaves, indicating that the expression of the PGβS-AS gene had enhanced the defense response of the plants toward the feeding larvae.
Figure 8.
Weights of tobacco hornworm larvae after feeding on leaves of wild type and on leaves of four different lines of PG βS-tobacco hornworm AS transgenic plants. Ten larvae were fed leaves from 30-d-old plants. Leaves from the transgenic line had small lesions but no necrosis. Leaves were replaced each day with similar leaves from each of the plants. The larvae were allowed to feed for 10 d and then weighed. Data are means ± sd; n = 6.
DISCUSSION
Among the wound-inducible genes in tomato leaves are those coding for a subfamily of PGcat enzymes and a novel PG β-subunit protein (DellaPenna et al., 1996; Bergey et al., 1999). The PGcat gene in leaves is thought to play a role in signaling the activation of defense-related genes in response to herbivore and pathogen attacks by releasing OGA elicitors from plant cell walls and the production of H202 (Orozco-Cárdenas et al., 2001). Although the PG-β-subunit gene is thought to regulate PGcat activity (DellaPenna et al., 1996), the levels of its mRNA in leaves is initially very low (Bergey et al., 1999). About 3 h after wounding tomato leaves, the mRNA levels of the β-subunit began to increase, when PGcat mRNA and enzyme activity are already rapidly increasing. The β-subunit mRNA levels continue to increases for 9 h, when the PGcat mRNA is maximal. At this time, a decrease in PG activity occurs, suggesting that the increased synthesis of the β-subunit protein is associated with the decreasing PGcat activity (Orozco-Cárdenas and Ryan, 1999).
To further examine the role of the β-subunit in tomato leaves, tomato plants were transformed with the tomato leaf β-subunit gene in the antisense orientation, called PGβS-AS. Gel blots from tomato antisense plants, shown in Figure 1, confirmed that transformed plants exhibited increased levels of expression of the gene, compared with the wild type. Three transformants (TA2, TA3, and TA4) having the highest levels of expression of the PGβS-AS gene were chosen for further study.
When growing the antisense plants to maturity, an early senescence-like appearance of the leaves was observed that was preceded by the formation of lesions on the upper and abaxial surfaces of the leaves (Fig. 2, C and D). The lesions, with time, became extensive and led to cell death (Fig. 2E). As the plants grew, the developing flowers also exhibited lesions that led to structural abnormalities (Fig. 2F) and eventually resulted in flower abscission and a total absence of fruit. These abnormalities suggested that the suppression of synthesis of the β-subunit in leaves and flowers resulted in an increase in PGcat activity that led to a breakdown of the structural integrity of leaves and flowers. Because PG degrades pectin, the effects were thought to be caused by cell wall modification with the concomitant release of pectic fragments that can induce biological effects, such as the enhancement of defense gene expression (Albersheim et al., 1992; Farmer and Ryan, 1992). It appears that the antisense expression of the β-subunit gene in leaf and floral tissue has released a suppressed PGcat activity that has resulted in the abnormalities seen in Figure 2. The elevated levels of PGcat activity in leaves of the antisense plants before and after wounding (Fig. 3) supports the hypothesis that the β-subunit protein negatively modulates PG-cat activity (DellaPenna et al., 1996).
Pectic fragments produced by PG are known to produce H2O2 in both cultured cells and in intact leaves (Low et al., 1996; Orozco-Cárdenas and Ryan, 1999; Orozco-Cárdenas et al., 2001). Histochemical assays to identify H2O2 in expanded leaves of the PGβS-AS plants visualized the accumulation of H2O2 in the lesions in the abaxial side of the leaves (Fig. 4A). The presence of H2O2 in the transgenic leaves was confirmed by directly measuring its concentrations in leaf extracts (Fig. 4B).
The elevated PGcat activity and H2O2 levels in the PG β-subunit antisense plants were similar to levels found in plants transformed with the prosystemin gene associated with signaling of synthesis of defense genes (Orozco-Cárdenas and Ryan, 1999). Leaves of antisense plants exhibited moderately elevated increases in mRNAs coding for both early and late signaling-associated genes over levels found in wild-type plants (0 time, Fig. 5). However, when wounded, the mRNA levels responded with kinetics that differed in two ways from wild-type plants. First, the early genes, including PGcat, were expressed within 1 h after wounding, as in wild-type plants, but then were maintained at higher levels 4 h after wounding (Fig. 5A) and beyond. Second, the late genes responded as if they were early genes, with the mRNAs accumulating at 1 h after wounding instead of at 4 h as in wild-type plants, and increased to higher levels than in wild-type plants (Fig. 5B). The rapid increases in late gene mRNAs were reflected in increases in proteinase Inh I and II proteins, which were elevated to much higher levels than could be induced in wild-type plants by wounding (Fig. 6). The proteinase inhibitor proteins were also induced to higher levels in leaves of transgenic plants over those of wild-type plants when treated with systemin, OGA, and JA (Fig. 7). These experiments indicated that the suppression of the β-subunit gene in tomato leaves was potentiating the induction of defense genes in response to wounding and elicitors.
The enhancement of the wound response in the PSβS-AS plants was shown to be biologically relevant by demonstrating that they had developed an increased resistance against the herbivore tobacco hornworm. Larvae feeding on transgenic leaves with local lesions for several days were less than one-half the size of larvae feeding on leaves from wild-type plants (Fig. 8). The increased resistance to tobacco hornworm larvae may be ascribed in large part to the rapid defense responses of the leaves (compare with Figs. 5 and 6) to insect feeding damage. The high levels of H2O2 generated in the lesions by pectic fragments may also be a contributing factor to the increased resistance (Felton et al., 1994; Orozco-Cárdenas et al., 2001). It is also possible that in addition to defense proteins, the synthesis of compounds leading to necrosis may have been occurring that could have contributed to the reduced feeding by the insects.
In tomato plants transformed with the prosystemin gene, PGcat activity and H2O2 are elevated constitutively in leaves (Orozco-Cárdenas and Ryan, 1999; Orozco-Cárdenas et al., 2001). However, in these plants, lesions on leaves are not present, and flowering and fruit development appear normal. Thus, processes associated with the increased levels of PGcat and H2O2 that are involved with necrosis, cell death, and flower abscission apparently are not activated in plants transformed with the prosystemin gene. The addition of OGA or H2O2 to plant cells also does not cause necrosis (Lecourieux et al., 2002). It appears that the constitutive loss of the β-subunit in the tomato plants has, over time, perturbed complex interactions that are usually associated with pathogen attacks that result in necrosis and cell death.
The abnormal increase in PGcat activity resulting from the expression of an antisense β-subunit gene in tomato plants has revealed that the regulation of the levels of β-subunit protein in vivo may play an important role in regulating PGcat activity in tissues and organs of the plants.
MATERIALS AND METHODS
Plant Transformation
Tomato (Lycopersicon esculentum cv Better Boy hybrid VFN) plants were transformed with a tomato leaf wound-inducible PG β-subunit gene in the antisense orientation. The chimeric gene was composed of a β-subunit cDNA (Bergey et al., 1999), under the control of the constitutive cauliflower mosaic virus 35S promoter, and terminated with the 3′ region of the T7 gene from Agrobacterium tumefaciens. An 1,800-bp fragment of the β-subunit cDNA was generated by restriction digestion with XhoI/BamHI and incorporated in its antisense orientation within the binary vector pART-27 (Gleave, 1992). The vector was introduced into A. tumefaciens LBA 4404, which was employed to transform tomato plants as described previously (Orozco-Cárdenas et al., 1993). Kanamycin-resistant transgenic tomato plants transformed with an empty pART-27 vector bearing the npt-II gene were also produced as a plant transformation control.
RNA Isolation and Gel-Blot Hybridization
Total RNA was extracted from random leaves of wild-type (untransformed) and four antisense transgenic tomato plants. The leaves from 6- to 8-week-old tomato plants were frozen in liquid nitrogen ground to a fine powder and stored at –80°C. Total leaf RNA was extracted using the Trizol reagent (Gibco-BRL, Rockville, MD) following the manufacturer's recommendations. Total RNA pellets were dissolved in 25 μL of RNase-free water and quantified spectrophotometrically. RNA quality was determined by gel fractionation in agarose/formaldehyde followed by ethidium bromide staining and UV visualization before analyzing for specific mRNAs (Sambrook et al., 1989). Total leaf RNA (20 μg) was fractionated by electrophoresis in 1.4% (w/v) agarose gels with formaldehyde, blotted onto nylon membranes, and incubated at 65°C for 1 h in hybridization buffer (5× sodium chloride/sodium phosphate/EDTA [SSPE; pH 7.4], 5× Denhardt's solution, 1% [w/v] SDS, and 10% [w/v] Dextran sulfate). Radioactive32P-dCTP-labeled probes were generated by random priming (DECA prime II kit, Ambion, Austin, TX) from tomato cDNAs encoding wound-inducible defense genes (Bergey et al., 1999). The genes include Inh I (Graham et al., 1985a), Inh II (Graham et al., 1985b), and CPI (Moura and Ryan, 2001). An 18S ribosomal RNA gene probe was used as a loading control. Synthetic oligonucleotide probes were purified using Bio-spin P6 chromatography columns (Bio-Rad, Hercules, CA). The probes were heat denatured, added to the hybridization buffer, and incubated with the blocked membranes overnight at 65°C. Membranes were washed once with 2× SSPE for 20 min at room temperature, two to three times with 2× SSPE and 1% (w/v) SDS for 15 to 30 min at 65°C, and then exposed for 15 to 32 h to x-ray film or to a PhosphorImager (Bio-Rad).
PG Assay
Extracts from leaves of wild-type and antisense transgenic tomato plants were prepared for PG assays as described previously (Bergey et al., 1999). In brief, 20 g of leaves was homogenized using a Sorvall Omnimizer (Sorvall, DuPont, Wilmington, DE) in 60 mL of ice-cold 0.1 m sodium citrate buffer (pH 6.0), containing 1 m NaCl, 4 mm ascorbic acid, 5 mm dithiotreitol, 2% (w/v) polyvinylpyrrolidone, and 0.1% (w/v) bovine serum albumin. The extracts were filtered through four layers of cheesecloth, incubated at 4°C for 3 h, and centrifuged at 10,000g for 20 min at 4°C. Proteins were precipitated at 4°C by the slow addition of solid ammonium sulfate to a final concentration of 80% (w/v) and stirred for 1 h at 4°C. The precipitated proteins were recovered by centrifugation at 10,000g for 20 min at 4°C, and the pellet was resuspended in 20 mL of 1 m NaCl and dialyzed against 1 m NaCl at 4°C for 24 h. Total PG activity was determined by incubating aliquots equivalent to 0.1 g fresh weight in 1 mL of reaction mixture containing 0.2 m NaCl and 0.1% (w/v) poly-GalUA (pH 4.5, Sigma) at 37°C and measuring reducing sugars by the arsenomolybdate method (Nelson, 1944). A unit of PG was defined as the amount that caused a 0.1 OD increase at 520 nm per 30 min at 37°C per 0.1 g fresh weight. All assays were repeated three times in duplicate.
Detection and Quantification of H2O2
H2O2 was detected cytochemically in the leaves of antisense transgenic tomato plants using the DAB technique, as previously described (Orozco-Cárdenas and Ryan, 1999). A 1 mg mL–1 solution of DAB (pH 4.5) was vacuum infiltrated into leaves of tomato plants at 25°C for 1 h; 1 h later, the developed color was photographed.
Quantification of H2O2 was performed by the method of Rao et al. (2000). Leaves from 6- to 8-week-old plants were frozen and ground to a powder under liquid nitrogen, incubated on ice for 5 min, and pelleted by centrifugation at 10,000g for 10 min at 4°C. The supernatant was adjusted to about pH 7.5 with 0.2 m NH4OH (pH 9.5) and briefly centrifuged at 3,000g for 2 min to sediment the insoluble material. Extracts were cleared by passing through columns of AG 1X-8 resin (ionic from chloride, Bio-Rad) followed by double-distilled water (Rao et al., 2000; Orozco-Cárdenas and Ryan, 2002).
H2O2 levels in the cleared extracts were quantified using an Amplex Red Hydrogen Peroxide Assay kit (Molecular Probes, Inc., Eugene, OR) following the manufacturer's recommendations. A 100-μL aliquot of each extract was placed in a microplate well with an equal volume of a solution containing 1 unit mL–1 of horseradish peroxidase in 50 mm sodium phosphate buffer (pH 7.4) and incubated for 30 min at room temperature. Fluorescence was measured with a fluorescence microplate reader (Perkin-Elmer, Ltd., Beaconsfield, Buckinghamshire, UK), using an excitation of 560 ± 5 nm and fluorescence emission of 590 ± 5 nm. The concentration of H2O2 in each sample was calculated using a standard curve obtained with known concentrations of pure H2O2.
Wounding and Elicitor Assays
Transformed plants were transferred to soil and analyzed for proteinase Inh I accumulation in the leaves in response to wounding and chemical elicitors four weeks later. The terminal leaflet of fully expanding leaves of control (wild type) and transgenic tomato plants were wounded twice with a hemostat across the midvein. Inh I protein levels in expressed leaf juice from wounded leaflets and from unwounded lateral leaflets from the same leaf were determined 24 h later.
To determine if Inh I protein could be induced by systemin and JA, leaves of wild-type and transgenic plants were excised with a razor blade at the base of the petiole and were allowed to imbibe a solution of systemin (25 nm) or JA (100 μm) or 250 μg mL–1 OGA for 30 min under light. The leaves were then transferred to vials with water and incubated 24 h at 25°C within plexiglas boxes under constant light (300 mE m–2 s–1). Proteinase inhibitors were quantified in expressed leaf extracts by radial immunoradial diffusion as described (Ryan, 1967; Trautman et al., 1971).
Tobacco Hornworm (Manduca sexta) Larvae Feeding Assays
Tobacco hornworm eggs and a hornworm artificial diet were obtained from Carolina Biological Supplies (Burlington, NC). The eggs were hatched as described by Howe et al. (1996). Newly hatched larvae were provided with an artificial diet for 3 d, and first instar larvae of uniform weight (about 8 mg) were selected. Ten tobacco hornworm larvae were placed in petri dishes together with excised leaves from 8-week-old wild-type control and transgenic tomato plants. The larvae were allowed to feed on the leaves for 10 d while maintained in a controlled environment at 25°C with constant light of 300 mE m–2 s–1).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.023226.
References
- Albersheim P, Darvill A, Augur C, Jong-Job C, Everhard S, Hahn M, Marfa V, Mohnen D, O'Neill MA, Spiro MD (1992) Oligosaccharins, oligosaccharide regulatory molecules. Accounts Chem Res 25: 77–82 [Google Scholar]
- Bergey DR, Howe GA, Ryan CA (1996) Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci USA 93: 12053–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey DR, Orozco-Cardenas ML, de Moura DS, Ryan CA (1999) A wound- and systemin-inducible polygalacturonase in tomato leaves. Proc Natl Acad Sci USA 96: 1756–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP (1999) Role of active oxygen species and NO in plant defense responses. Curr Opin Plant Biol 2: 287–294 [DOI] [PubMed] [Google Scholar]
- Brady CJ, MacAlpine G, McGlasson WB, Ueda Y (1982) Polygalacturonase in tomato fruits and the induction of ripening. Aust J Plant Physiol 9: 171–178 [Google Scholar]
- DellaPenna D, Kates DS, Bennett AB (1987) Polygalacturonase gene expression in Rutgers, rin nor and Nr tomato fruits. Plant Physiol 85: 502–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D, Lashbrook CC, Toenjes K, Giovannoni JJ, Fischer RL, Bennett AB (1990) Polygalacturonase isozymes and pectin depolymerization in transgenic rin tomato fruit. Plant Physiol 94: 1882–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D, Lincoln JE, Fischer RL, Bennett AB (1989) Transcriptional analysis of polygalacturonase and other ripening associated genes in Rutgers, rin, nor and Nr tomato fruit. Plant Physiol 90: 1372–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D, Watson C, Liu J, Schuchman D (1996) The β subunit of tomato fruit Polygalacturonase Isoenzyme 1 defines a new class of plant cell proteins involved in pectin metabolism: AroGPs (Aromatic Amino Acid Rich Glyco Proteins). In J Visser, AGJ Boragen, eds, Pectins and Pectinesterases. Elsevier Science, Amsterdam, pp 247–252
- Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton GW, Summers CB, Mueller AJ (1994) Oxidative responses in soybean foliage to herbivory by bean leaf beetle and three-cornered alfalfa hopper. J Chem Ecol 20: 639–649 [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL (1989) Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell 1: 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave AP (1992) A versatile binary vector system with a T-DNA organizational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20: 1203–1207 [DOI] [PubMed] [Google Scholar]
- Graham JS, Pearce G, Merryweather J, Titani K, Ericsson L, Ryan CA (1985a) Wound-induced proteinase inhibitors from tomato leaves: I. The cDNA deduced primary structure of pre-inhibitor I and its posttranslational processing J Biol Chem 260: 6555–6560 [PubMed] [Google Scholar]
- Graham JS, Pearce G, Merryweather J, Titani K, Ericsson LH, Ryan CA (1985b) Wound-induced proteinase inhibitor mRNA from tomato leaves: II. The cDNA deduced primary sequence of pre-inhibitor II. J Biol Chem 260: 6561–6564 [PubMed] [Google Scholar]
- Grierson D, Maunders MJ, Holdsworth MJ, Ray J, Bird C, Moureau P, Schuch W, Slater A, Knapp JE, Tucker GA (1987) Expression and function of ripening genes. In D Nevins, R Jones, eds, Tomato Biotechnology. Alan R Liss, Inc., New York, pp 309–323
- Handfiel KA, Bennett AB (1998) Polygalacturonases: many genes in search of a function. Plant Physiol 117: 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CY, Wang PJ (1983) Meristem, shoot tip and bud cultures. In DF Evans, WR Sharp, PV Ammirato, Y Yamada, eds, Handbook of Plant Cell Culture: Techniques for Propagation and Breeding, Vol. 1. Macmillan Publishing, New York, pp 127–129 [Google Scholar]
- Kalaitzis P, Solomos T, Tucker ML (1997) Three different polygalacturonases are expressed in tomato leaf and flower abscission, each with a different temporal expression pattern. Plant Physiol 113: 1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E, Vemeer E, Bruinsma J (1988) Conversion of the polygalacturonase isoenzymes from ripening tomato fruits. Physiol Plant 72: 108–114 [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Mol Biol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14: 2627–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Williams MM, Loh YT, Lee GI, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130: 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PS, Merida JR (1996) The oxidative burst in plant defense: function and signal transduction. Plant Physiol 96: 533–542 [Google Scholar]
- McGurl B, Orozco-Cardenas ML, Pearce G, Ryan CA (1994) Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc Natl Acad Sci USA 91: 9799–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosrefi M, Luh BS (1983) Carbohydrate composition and electrophoretic properties of tomato polygalacturonase isoenzymes. Eur J Biochem 135: 511–514 [DOI] [PubMed] [Google Scholar]
- Moura DS, Bergey D, Ryan CA (2001) Characterization and localization of a wound-inducible type I serine-carboxypeptidase from leaves of tomato plants (Lycopersicon esculentum Mill.). Planta 212: 222–230 [DOI] [PubMed] [Google Scholar]
- Muñoz JA, Coronado C, Perez-Hormaeche J, Kondorosi A, Ratet P, Palomares AJ (1998) MsPG3 a Medicago sativa polygalacturonase gene expressed during the alfalfa-Rhizobium meliloti interaction. Proc Natl Acad Sci USA 95: 9687–9692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153: 375–380 [Google Scholar]
- Orozco-Cárdenas ML, McGurl B, Ryan CA (1993) Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc Natl Acad Sci USA 90: 8273–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide behaves as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin and methyl jasmonate. Plant Cell 13: 179–191 [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96: 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Ryan CA (2002) Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol 130: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, Sander L, Child R, Onckelen HV, Ulvskov P, Bernhard B (1996) Isolation and characterization of a pod dehiscence zone-specific polygalacturonase from Brassica napus. Plant Mol Biol 31: 517–527 [DOI] [PubMed] [Google Scholar]
- Pogson BJ, Brady CJ, Orr GR (1991) On the occurrence and structure of subunits of endopolygalacturonase isoforms in mature-green and ripening tomato fruits. Aust J Plant Physiol 18: 65–79 [Google Scholar]
- Pressey R (1991) Polygalacturonase in tree pollens. Phytochemistry 30: 1753–1755 [Google Scholar]
- Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR (2000) Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA (1967) The quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem 19: 434–440 [DOI] [PubMed] [Google Scholar]
- Ryan CA, Farmer EE (1991) Oligosaccharide signals in plants: a current assessment. Annu Rev Plant Physiol Mol Biol 42: 651–670 [Google Scholar]
- Sambrook J, Fristch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory, Cold Sprint Harbor, New York
- Sitrit Y, Hadfield KA, Bennett AB, Bradford KJ, Downie AB (1999) Expression of a polygalacturonase associated with tomato seed germination. Plant Physiol 121: 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer DJ, Chin CK (1984) Meristem and shoot-tip culture for propagation, pathogen elimination and germplasm preservation. Hortic Rev 5: 221–277 [Google Scholar]
- Trautman R, Cowan KM, Wagner GG (1971) Data processing for radial immunodiffusion. Immunochemistry 8: 901–916 [DOI] [PubMed] [Google Scholar]
- Wang PJ, Arntzen C (1991) Micropropagation through meristem culture. In YPS Bajaj, ed, Biotechnology in Agriculture and Forestry, High-Tech and Micropropagation I, Vol. 17. Springer-Verlag, Berlin, pp 32–34 [Google Scholar]
- Watson CF, Zheng L, DellaPenna D (1994) Reduction to tomato polygalacturonase β subunit expression affects pectin solubilization and degradation during fruit ripening. Plant Cell 6: 1623–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Heupel RC, DellaPenna D (1992) The β subunit of tomato fruit polygalacturonase isoenzyme 1: Isolation, characterization, and identification of unique structural features. Plant Cell 4: 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]