Abstract
Recognition of self-pollen during the self-incompatibility response in Brassica oleracea is mediated by the binding of a secreted peptide (the S locus cysteine-rich protein) to the S locus receptor kinase (SRK), a member of the plant receptor kinase (PRK) superfamily. Here, we describe the characterization of three proteins that interact with the cytosolic kinase domain of SRK. A B. oleracea homolog of Arabidopsis kinase-associated protein phosphatase was shown to interact with and dephosphorylate SRK and was itself phosphorylated by SRK. Yeast (Saccharomyces cerevisiae) two-hybrid screens identified two additional interactors, calmodulin and a sorting nexin, both of which have been implicated in receptor kinase down-regulation in animals. A calmodulin-binding site was identified in sub-domain VIa of the SRK kinase domain. The binding site is conserved and functional in several other members of the PRK family. The sorting nexin also interacted with diverse members of the PRK family, suggesting that all three of the interacting proteins described here may play a general role in signal transduction by this family of proteins.
Plant genomes encode large numbers of receptors with Ser/Thr protein kinase activity. These plant receptor kinases (PRKs) are related to, but phylogenetically distinct from, the receptor Tyr kinase (RTK) and receptor Ser/Thr kinase (RSK) families in animals, suggesting that the three families have evolved independently from a common ancestor (Shiu and Bleecker, 2001). The independent evolution of these gene families is reflected in the fact that, to date, none of the proteins that have been shown to interact with PRKs are closely homologous to RTK- and RSK-interacting proteins. However, despite the many differences between plant and animal receptor kinase systems, there are indications that they function in a similar manner with, for example, unrelated interacting proteins carrying out analogous roles in the two kingdoms (Cock et al., 2002).
One of the best characterized PRKs is the S locus receptor kinase (SRK). SRK is located in the plasma membrane of the stigmatic papillar cells and is the female component of the self-incompatibility (SI) response in Brassica spp. (Stein et al., 1991; Delorme et al., 1995; Takasaki et al., 2000). The SI response allows the recognition and rejection of self-pollen grains on the stigma surface and, hence, promotes outcrossing (Cock, 2000). There is now strong evidence that SRK mediates self-pollen recognition by binding to a small, secreted peptide, S locus Cys rich (SCR), located in the pollen coat (Schopfer et al., 1999; Takayama et al., 2000, 2001; Shiba et al., 2001; Kachroo et al., 2001).
SRK is phosphorylated after an incompatible pollination (Cabrillac et al., 2001), and in vitro experiments indicate that activation of SRK may involve autophosphorylation between SRK molecules associated in the membrane (Giranton et al., 2000). This suggests that SRK is activated by a mechanism similar to that described for animal receptor kinases (Heldin, 1995).
The signal transduction pathway downstream of SRK has been partially characterized. ARC1 (arm repeat containing 1; a complex protein with multiple domains including a Leu zipper, a U box, and a C-terminal Arm repeat domain; accession no. AJ427335) interacts with the SRK kinase domain and is required for efficient rejection of self-pollen (Gu et al., 1998; Stone et al., 1999). ARC1, which has E3 ubiquitin ligase activity, is thought to mediate pollen rejection by promoting the ubiquitination of compatibility factors in the pistil (Stone et al., 2003).
There are also indications that negative control of SRK signaling may play a role in SI signaling, both in the basal state, before pollination, and after SRK activation. SRK interacts with two thioredoxin h-like proteins (THL1 and THL2; Bower et al., 1996). THL1 inhibits constitutive autophosphorylation of SRK in the absence of ligand (Cabrillac et al., 2001), and its interaction with SRK requires the presence of a conserved Cys residue on the cytoplasmic side of the SRK transmembrane domain (Mazzurco et al., 2001). Therefore, THL1 appears to act as a basal state inhibitor, in a manner analogous to FKBP12, which binds to and prevents activation of transforming growth factor β receptor II (Wang et al., 1996).
SRK also interacts, in vitro, with Arabidopsis kinase-associated protein phosphatase (KAPP; accession no. AJ427336; Braun et al., 1997). KAPP interacts with many other PRKs (Braun et al., 1997; Williams et al., 1997; Stone et al., 1998; van der Knaap et al., 1999; Gomez-Gomez et al., 2001); for example, KAPP is phosphorylated by CLV1 (CLAVATA1) and dephosphorylates the kinase domain of this receptor in vitro (Williams et al., 1997; Stone et al., 1998). Experiments in which KAPP expression has been manipulated in transgenic plants indicate that KAPP is a negative regulator of both CLV1 and FLS2, and it has been proposed that KAPP has a general role in the down-regulation of a large spectrum of PRKs (Williams et al., 1997; Stone et al., 1998; Gomez-Gomez et al., 2001). Endocytosis is known to occur in plants (Emans et al., 2002), and KAPP may also have an important role in regulating PRK internalization. When the PRK AtSERK1 was transiently co-expressed with KAPP, the former was sequestered in intracellular vesicles (Shah et al., 2002).
Down-regulation and internalization by endocytosis are essential for correct regulation of receptor kinases in animals, and a wide range of mechanisms have been described, including inhibitors acting on receptors in their basal state; regulation by dephosphorylation, by phosphorylation, or by calmodulin binding; Cbl-mediated receptor ubiquitylation; and sorting nexin-mediated targeting to endosomes (San José et al., 1992; Kurten et al., 1996; Wang et al., 1996; Martín-Nieto and Villalobo, 1998; Feinmesser et al., 1999; Carraway III and Sweeney, 2001; Östman and Böhmer, 2001). Moreover, individual receptors may be regulated by several of these processes.
We show here that SRK interacts with and is dephosphorylated by the Brassica homolog of KAPP, indicating that this protein plays a role in SRK down-regulation. SRK was also shown to interact with calmodulin and a sorting nexin, both of which are proteins that have been implicated in receptor kinase down-regulation in animals. KAPP, calmodulin, and the sorting nexin also interact with additional, diverse PRKs. The possible roles of these three proteins in PRK down-regulation and endocytosis are discussed.
RESULTS
SRK Interacts with a Brassica oleracea Homolog of KAPP
The demonstration that the Arabidopsis KAPP protein interacts with SRK in vitro (Braun et al., 1997) suggested that a similar interaction may occur in stigmas between SRK and a Brassica homolog of KAPP. A B. oleracea KAPP homolog was isolated from a stigma cDNA library and shown to share 80.1% similarity overall with Arabidopsis KAPP at the amino acid level. Interaction between Arabidopsis KAPP and PRKs is mediated by a forkhead-associated (FHA) domain (Li et al., 1999). The Brassica KAPP FHA domain is highly similar to that of Arabidopsis KAPP, including the presence of four highly conserved residues that are required for binding to phosphoproteins (Fig. 1A).
Figure 1.
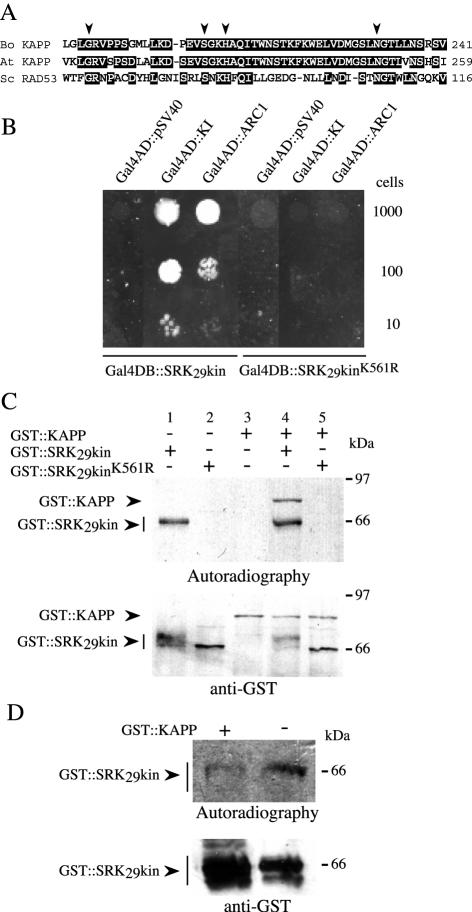
Interaction of the Brassica KAPP and SRK proteins. A, Alignment of the FHA domains from the Brassica (Bo KAPP) and Arabidopsis (At KAPP) KAPP proteins and yeast (Saccharomyces cerevisiae) RAD53 (Sc RAD53). Arrowheads indicate residues required for binding of Arabidopsis KAPP to PRKs (Li et al., 1999). B, Kinase interaction (KI) domain of Brassica KAPP and the C-terminal end of ARC1 (containing the Arm repeats) interact with the wild type but not with a kinase-inactive form of the SRK29 kinase domain in the yeast two-hybrid assay. Different numbers of yeast strain PJ69-4A cells expressing the prey and bait indicated above and below the panel, respectively, were inoculated by spotting onto selective medium lacking His and adenine and grown at 30°C for 5 d. C, Phosphorylation of Brassica KAPP by SRK. Glutathione S-transferase (GST) fusion proteins with Brassica KAPP or with either wild-type or mutant forms of the SRK29 kinase domain were mixed in phosphorylation reactions as indicated. The KAPP and SRK fusion proteins are indicated by arrowheads. Proteins were detected by autoradiography (top) or by immunoblotting with an anti-GST antibody (bottom). Note that the active form of the SRK29 kinase domain was detected as a diffuse band (indicated by a bar). Phosphatase treatment showed that this was due to the presence of differentially phosphorylated isoforms (data not shown). D, Dephosphorylation of SRK by KAPP. Radiolabeled, phosphorylated GST::SRK29kin was incubated in the presence or absence of GST::KAPP, and the proteins were separated on an SDS-PAGE gel. Radiolabeled GST::SRK29kin was detected by autoradiography (top) and total GST::SRK29kin protein was detected with an anti-GST antibody (bottom).
We used the yeast two-hybrid system to test whether Brassica KAPP interacts with the kinase domain of SRK. The KI domain of Brassica KAPP was expressed as a fusion protein with the activation domain of Gal4 (Gal4AD::KI) in yeast cells that also expressed the kinase domain of SRK29 fused to the DNA-binding domain of Gal4 (Gal4DB::SRK29kin). As a positive control for this experiment, we tested whether the kinase domain of SRK interacted with ARC1 in this system. For this, we cloned the Arm-repeat region of a B. oleracea homolog of ARC1 (residues 362–661) and expressed it as a fusion protein with Gal4 (Gal4AD::ARC) in the same kinase domain-expressing yeast strain. ARC1 has been shown to interact with SRK in closely related B. napus (Gu et al., 1998). Both combinations of interactors were able to induce all three reporter genes in the PJ69-4A strain (James et al., 1996), leading to His and adenine prototrophy (Fig. 1B) and production of α-galactosidase enzyme activity (data not shown). In contrast, neither the KAPP nor the ARC1 fusion proteins interacted with an enzymatically inactive, mutant form of the SRK29 kinase domain (Gal4DB::SRK29kinK561R; Fig. 1B). These observations corroborate previous studies that have shown that ARC1 and Arabidopsis KAPP interact only with the phosphorylated form of the SRK kinase domain (Braun et al., 1997; Gu et al., 1998).
To test whether KAPP could be phosphorylated by SRK, a fusion protein consisting of GST fused to Brassica KAPP lacking its predicted N-terminal signal anchor (residues 1–47) was expressed in Escherichia coli, and the purified protein (GST::KAPP) was added to a phosphorylation reaction in the presence of either the wild type or a mutant form of the SRK29 kinase domain that had also been expressed in E. coli as protein fusions with GST (GST::SRK29kin and GST::SRK29kinK561R). KAPP was phosphorylated by the wild-type SRK29 kinase but not by the kinase-negative mutant form (Fig. 1C). A phosphorylation assay confirmed that the wild-type kinase domain construct, GST::SRK29kin, was capable of autophosphorylation in vitro, whereas the mutant form, GST::SRK29kinK561R, was enzymatically inactive (data not shown).
Furthermore, Figure 1D shows that when GST::KAPP was added to radiolabeled, phosphorylated GST::SRK29kin in vitro, the latter was dephosphorylated, indicating that SRK is a substrate for the phosphatase activity of KAPP.
Identification of Novel SRK-Interacting Proteins Using the Yeast Two-Hybrid System
The above experiments showed that the kinase domain of SRK29 was able to interact specifically with KAPP and ARC1 in the two-hybrid assay. Based on this result, we initiated a screen to identify novel interacting partners using the two-hybrid system. Several two-hybrid screens were performed, using both wild-type (Gal4DB::SRK29kin) and mutant (Gal4DB::SRK29kinK561R) forms of the SRK kinase domain and the kinase domain of SFR1 (Gal4DB::SFR1kin), a receptor closely related to SRK that is also expressed in stigmas (Pastuglia et al., 2002). After each screen, potential interactors were co-expressed with a fusion between murine p53 (residues 72–390) and the Gal4 DNA-binding domain (Gal4DB::p53) to eliminate nonspecific interactions. Four novel interactors were identified in these screens, the most interesting being calmodulin and a Brassica SNX1 (sorting nexin 1; accession no. AJ441072) homolog (Table I). Calmodulin cDNAs were recovered in screens with all three bait proteins, and the interaction was confirmed in a second yeast strain, Y190, using both wild-type and mutant SRK kinase domain constructs (data not shown).
Table I.
Interaction of ARC1, KAPP, and calmodulin with the kinase domains of SRK and SFR1 in the yeast two-hybrid system
| Bait: Prey | Gal4DB:: SRK29kin | Gal4DB:: SRK29kinK561R | Gal4DB:: SFR1kin | Gal4DB:: p53 |
|---|---|---|---|---|
| Gal4AD::ARC1 | + | - | +a | - |
| Gal4AD::KAPP | + | - | + | - |
| Gal4AD::calmodulin | +/-a | +a | +a | - |
| Gal4AD::SNX1 | - | +a | + | - |
| Gal4AD::pSV40 | - | - | - | + |
a These interactions were also observed when the stigma cDNA library was screened with the corresponding bait construction.
The calmodulin cDNAs identified in the three screens corresponded to two different genes (designated Calmodulin 1, accession no. AJ427337; and Calmodulin 2, accession no. AJ427338) that differed significantly in their 3′ non-translated regions. However, the coding regions of these two calmodulin genes are 93.2% identical, and they encode identical proteins. The deduced amino acid sequence of the Brassica calmodulins is 98.0% identical to that of a previously described B. napus calmodulin (accession no. AF150059).
SRK Interacts with Calmodulin in Vitro
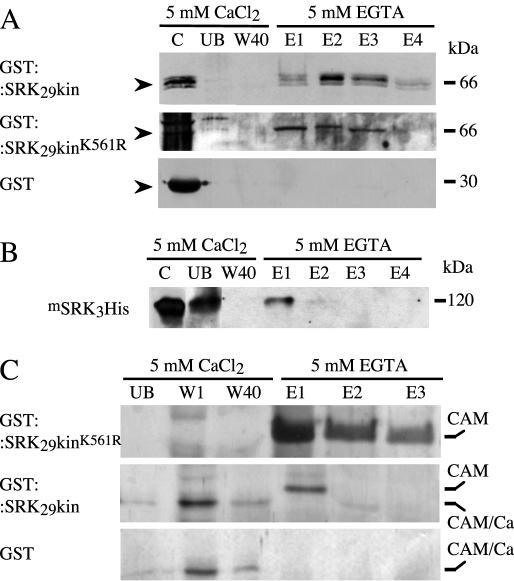
Calmodulin-Sepharose affinity binding was carried out to confirm the interaction between calmodulin and the kinase domain of SRK. Figure 2A shows that fusion proteins consisting of GST plus either the wild type or a mutant form of the SRK29 kinase domain bound to calmodulin-Sepharose beads in the presence of 5 mm CaCl2 and were eluted from the beads when the 5 mm CaCl2 was replaced by 5 mm EGTA. When GST alone was loaded on the column, some protein bound to the beads, but this was removed by washing with 5 mm CaCl2 and was not specifically eluted by 5 mm EGTA.
Figure 2.
Calmodulin binds to the kinase domain of SRK in vitro. A, Binding of GST or the GST protein fused with either wild-type or mutant forms of the SRK29 kinase domain to calmodulin-Sepharose beads. GST-containing proteins were detected with an anti-GST antibody. B, Binding of the integral SRK protein to calmodulin-Sepharose beads. A recombinant hexa-His epitope-tagged, kinase-inactive form of SRK (mSRK3His; Giranton et al., 2000) was used for this experiment. SRK protein was detected with the monoclonal antibody MAb85-36-71 (Giranton et al., 2000). C, Binding of Brassica calmodulin to glutathione-Sepharose beads carrying either GST or GST protein fused to either wild-type or mutant forms of the SRK29 kinase domain. Eluted proteins were detected by silver staining. Note that calmodulin alters its conformation in the presence of calcium resulting in a change in mobility. CAM, Calmodulin with no bound Ca2+; CAM/Ca, calmodulin with bound Ca2+; C, purified fusion protein; UB, unbound fraction; W1 and W40, washes; E1 to E4, eluted fractions. C and UB, One-quarter of the amount of protein loaded on and unbound to the column, respectively.
Similarly, when a kinase-negative, hexa-His epitope-tagged form of the integral SRK3 protein (mSRK3His) expressed in insect cells was subjected to calmodulin-Sepharose affinity binding, the recombinant protein bound to the calmodulin-Sepharose beads and was eluted in the presence of 5 mm EGTA (Fig. 2B).
The interaction between the kinase domain of SRK and calmodulin was also confirmed using an alternative affinity-binding approach. GST alone or fused to either the wild type or the mutant form of the SRK29 kinase domain was bound to glutathione-Sepharose beads. Brassica calmodulin 2 tagged with a hexa-His epitope was then expressed in insect cells, affinity purified on nickel-nitrilotriacetic acid agarose beads, and applied to beads carrying the SRK fusion proteins in the presence of calcium. After washing, calmodulin was specifically eluted from the beads in the presence of 5 mm EGTA. Figure 2C shows that calmodulin bound to beads carrying both wild-type and mutant kinase domain protein fusions but not to beads carrying GST alone.
Calmodulin Interacts with an Amphiphilic Helix in Sub-Domain VIa of the SRK Kinase Domain
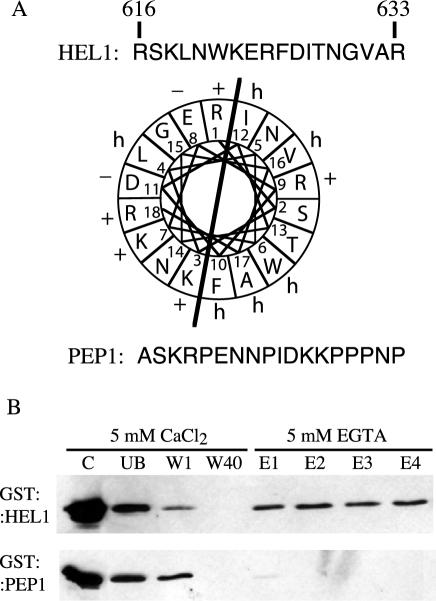
Calmodulin has been shown to interact with its target proteins by binding to small amphiphilic α-helices (Erickson-Viitanen and DeGrado, 1987). To identify the region of SRK responsible for the interaction with calmodulin, we searched for small amphiphilic α-helices in the SRK kinase domain using multiple secondary structure prediction programs. Two small potential helices were found in subdomains VIa and VII (for a definition of the kinase sub-domains, see Hanks et al., 1988). Comparison of all the SRK alleles available in the databases showed that all of the deduced proteins were predicted to possess an amphiphilic helix at the same position in sub-domain VIa. Therefore, this region, designated HEL1, was selected for further study. The HEL1 region of the SRK3 protein (amino acids 617–643 of SRK3) is shown in Figure 3A as a helical projection. The amphiphilic nature of this region is indicated by the black bar.
Figure 3.
Calmodulin binds to sub-domain VIa of the kinase domain of SRK in vitro. A, Amino acid sequence of the HEL1 region of sub-domain VIa of SRK3. The wheel projection shows the amphiphilic nature of HEL1. The sequence of the control peptide PEP1 is also shown. B, Binding of GST::HEL1 and GST::PEP1 fusion proteins to calmodulin-Sepharose beads. GST-containing proteins were detected with an anti-GST antibody. C, purified GST fusion protein; UB, unbound fraction; W1 and W40, first and last washes; E1 to E4, eluted fractions.
Figure 3B shows that, when the HEL1 peptide was fused to GST (GST::HEL1), the fusion protein bound to calmodulin-Sepharose beads and was eluted in the presence of 5 mm EGTA, indicating that HEL1 interacts specifically with calmodulin. GST fused to a non-helical, nonamphiphilic peptide of the same size as, and of similar hydrophilicity to, HEL1 (PEP1 in Fig. 3A) did not bind to calmodulin-Sepharose beads (Fig. 3B).
Calmodulin Interacts with the Kinase Domains of a Wide Range of PRKs
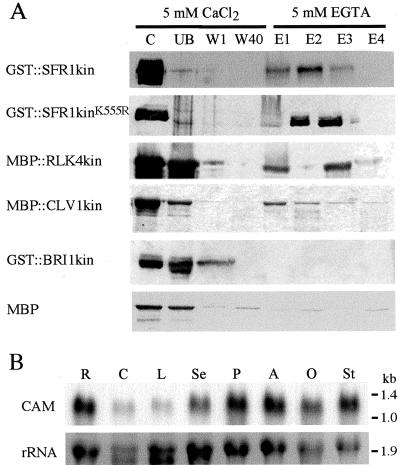
The fact that calmodulin interacted with the kinase domains of both SRK and SFR1 in the yeast two-hybrid assay (Table I) indicated that this interaction was not specific to SRK and suggested that calmodulin may interact with other receptor kinases. To test this, we expressed both wild-type and mutant forms of the SFR1 kinase domain and wild-type kinase domains of three additional members of the PRK superfamily, RLK4 (receptor-like kinase 4), CLV1, and BRI1 (brassinosteroid insensitive 1) as fusion proteins with either GST or the maltose-binding protein (MBP). RLK4 is a member of the S gene family of receptor kinases but is distantly related to SRK (approximately 25% amino acid identity depending on the allele of SRK); CLV1 and BRI1 are members of the family of PRKs that contain Leu-rich repeats in their extracellular domains. All five of the recombinant proteins were then purified and subjected to a calmodulin affinity-binding assay. Figure 4A shows that all the proteins tested, apart from GST::BRI1kin, bound to the calmodulin-Sepharose beads in the presence of 5 mm CaCl2 and were eluted after addition of 5 mm EGTA. Analysis of the sub-domain VIa regions of these proteins showed that all except BRI1 were predicted to include amphiphilic helices. Therefore, the presence of a predicted amphiphilic helix in kinase sub-domain VIa was correlated with the ability to bind calmodulin.
Figure 4.
Calmodulin binds to the kinase domains of SFR1, RLK4, and CLV1, but not BRI1, in vitro. A, Analysis of the binding of the kinase domains of SFR1, RLK4, CLV1, and BRI1 to calmodulin-Sepharose. The kinase domains were expressed as fusion proteins with either GST or MBP. Both a wild-type (GST::SFR1kin) and a kinase-inactive mutant form (GST::SFR1kinK555R) of the SFR1 kinase domain were tested. GST- and MBP-containing proteins were detected with anti-GST and anti-MBP antibodies, respectively. B, Abundance of calmodulin transcripts in different Brassica organs. Calmodulin transcripts were detected with a probe corresponding to the entire coding sequence. Lower, Ethidium bromide-stained rRNA. The positions of RNA size markers are shown at right in kilobase pairs. R, Root; C, cotyledon; L, leaf; Se, sepal; P, petal; A, anther; O, ovary; St, stigma.
Interestingly, we consistently observed that MBP::RLK4kin was eluted from the calmodulin-Sepharose beads in two peaks, indicating the presence of more than one calmodulin-binding site in the RLK4 kinase domain, the sites having different binding affinities.
The expression pattern of calmodulin was consistent with it interacting with multiple PRKs expressed in different parts of the plant because transcripts were detected in a wide range of organs (Fig. 4B).
Calmodulin and SRK Autophosphorylation
We were interested in determining whether calmodulin binding modified SRK autophosphorylation because HEL1 is located close to the activation loop in the kinase domain of SRK. In a kinase assay carried out in vitro, addition of calmodulin in the presence of 5 mm CaCl2 did not prevent autophosphorylation of GST::SRK29kin, and calmodulin was not phosphorylated by the kinase domain fusion protein (data not shown). The same result was obtained when the experiment was repeated with full-length SRK protein that had been expressed in insect cells (data not shown). This result suggests that calmodulin binding has no direct effect on SRK kinase activity. Again, calmodulin was not phosphorylated in these reactions, indicating that it is not a substrate of SRK kinase activity.
Interaction of SRK with a Sorting Nexin
Sorting nexins have been implicated in cargo trafficking through the endosomal system in mammals and yeast, and several members of this family interact with animal receptor kinases (Kurten et al., 1996; Haft et al., 1998; Parks et al., 2001; Teasdale et al., 2001). Therefore, we were particularly interested when a Brassica sorting nexin homolog (Brassica SNX1) was isolated as an interactor with the mutant form of the SRK29 kinase domain in the yeast two-hybrid screen (Table I).
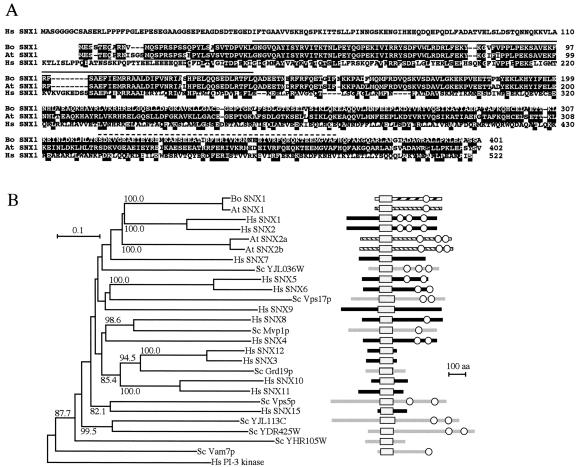
Sequence analysis showed that Brassica SNX1 was most similar to an SNX1 homolog from Arabidopsis but also shared extensive similarity with human (Homo sapiens) SNX1 (Fig. 5A). Human SNX1 contains a PX domain plus three predicted coiled-coil domains in the C-terminal part of the protein. Comparison of the PX domains of sorting nexins has allowed the identification of three highly conserved motifs, two of which (RRY/FSD/EFxxLxxxL and RR/KxxLxxY/F where x is any amino acid) are predicted to form a basic pocket involved in phosphoinositol binding and a third, Pro-rich motif (PPxPxK), which may be involved in protein-protein interactions (Sato et al., 2001; Xu et al., 2001). All three of these motifs are present in Brassica SNX1. Moreover, the COILS algorithm (Lupas, 1996) predicts a coiled-coil domain between residues 320 and 370 of Brassica SNX1, and the BLAST algorithm (Altschul et al., 1990) is able to match this region to human SNX1 in database searches (Fig. 5A). Hence, there are extensive similarities between human SNX1 and Brassica SNX1, suggesting that these two proteins carry out similar functions.
Figure 5.
Sequence analysis of Brassica SNX1 and the sorting nexin family in Arabidopsis. A, Alignment of Brassica SNX1 (Bo SNX1) with Arabidopsis (At SNX1) and human (Hs SNX1) SNX1. Residues conserved in at least two proteins are shaded. The phox homology (PX) and coiled-coil domains of Brassica SNX1 are overlined with a continuous and a dotted line, respectively. B, Neighbor-joining tree based on an alignment of the PX domains of Brassica SNX1 and of the Arabidopsis, human, and yeast sorting nexin families. The tree was constructed using the PAM250 amino acid substitution matrix. Numbers next to the branches are bootstrap values expressed as percentage confidence level and based on 1,000 repeats. The PX domain of human PI-3 kinase was used to root the tree. The PX domain polypeptide sequences compared were Brassica SNX1, Arabidopsis SNX1 (At SNX1; accession no. At5g06140), SNX2a (At SNX2a; At5g58440), and SNX2b (At SNX2b; At5g07120), several human (Hs SNX1, Q13596; Hs SNX2, O60749; Hs SNX3, O60493; Hs SNX4, O95219; Hs SNX5, Q9Y5X3; Hs SNX6, Q9UNH7; Hs SNX7, Q9UNH6; Hs SNX8, Q9Y5X2; Hs SNX9, Q9Y5X1; Hs SNX10, Q9Y5X0; Hs SNX11, Q9Y5W9; Hs SNX12, Q9UMY4; Hs SNX15, Q9NRS6), and yeast (Sc YJL036W, S56808; Sc Vps17p, NP_014775; Sc Mvp1p, P40959; Sc Grd19p, Q08826; Sc Vps5p, Q92331; Sc YJL113C, CAA98681; Sc YDR425W, S69707; Sc YHR105W, S48947; Sc Vam7p, P32912) sorting nexins and human PI-3 kinase (Hs PI-3 kinase, JC5500). The domain architecture of the sorting nexins is shown at right. Gray boxes, PX domains; white circles, coiled-coil domains.
Both the human and yeast genomes contain moderately large families of sorting nexins (Teasdale et al., 2001; Xu et al., 2001), but only three genes in the Arabidopsis genome have been annotated as sorting nexins. We extensively screened the Arabidopsis genome with all the available human and yeast sorting nexin sequences using the tBLASTn algorithm but did not identify any additional homologs. Therefore, the sorting nexin family in Arabidopsis is smaller and less diverse than in humans or in yeast (Fig. 5B).
To confirm the interaction between SRK and Brassica SNX1, GST alone or GST fused to wild-type or kinase-negative forms of the SRK29 kinase domain were bound to glutathione-Sepharose beads and then incubated with hexa-His-tagged SNX1. Kinase/SNX1 complexes were then eluted from the resin by addition of glutathione. Figure 6A shows that SNX1 was eluted from the resin only if kinase domain fusion proteins were present as bait. No SNX1 was detected in the glutathione elution fractions if the bait used was GST protein alone. Deletion analysis demonstrated that the PX domain of Brassica SNX1 was sufficient to mediate the interaction with SRK (Fig. 6B).
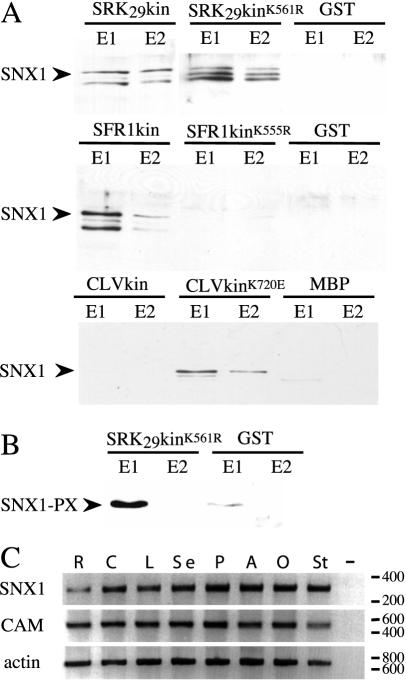
Figure 6.
Brassica SNX1 binds to the kinase domains of SRK, SFR1, and CLV1 in vitro. A, Elution of SNX1 from affinity resins loaded with GST or MBP proteins alone or GST or MBP fused with the kinase domains of SRK29, SFR1, or CLV1. Both wild-type and kinase-inactive mutant forms of the SRK29, SFR1, and CLV1 kinase domains were tested. B, Interaction between the SRK kinase domain and the PX domain (amino acids 1–157) of Brassica SNX1. Hexa-His-tagged SNX1 was detected with an anti-HIS antibody. In some cases, multiple bands were detected presumably due to the presence of either breakdown products or incompletely synthesized recombinant SNX1. E1 and E2, Eluted fractions. SRK29kin, GST::SRK29kin; SRK29kinK561R, GST::SRK29kinK561R; SFR1kin, GST::SFR1kin; SFR1kinK555R, GST::SFR1kinK555R; CLV1kin MBP::CLV1kin; CLV1kinK720E, MBP::CLV1kinK720E. C, RT-PCR analysis of SNX1 expression. Calmodulin (CAM) and actin cDNA were also amplified as controls. R, Root; C, cotyledon; L, leaf; Se, sepal; P, petal; A, anther; O, ovary; St, stigma; –, no cDNA control.
Using the same approach, we were also able to demonstrate interactions between SNX1 and two other PRK kinase domains, from SFR1 and CLV1 (Fig. 6A). SNX1 interacted with both wild-type and kinase-negative forms of the SRK kinase domain, but this was not the case for SFR1 and CLV1 where only the wild-type or only the kinase negative forms interacted, respectively. It is not clear why such differences were observed, but the differences were clearly reproducible. The interaction of SNX1 with the kinase domains of three diverse receptors suggests that this protein may play a role in multiple PRK signal transduction pathways. This hypothesis is supported by the fact that SNX1 transcripts were detected in a wide range of Brassica organs (Fig. 6C).
DISCUSSION
In this study, we have investigated interactions between several cytoplasmic stigma proteins and the kinase domain of SRK. We show that a stigma-expressed Brassica homolog of KAPP interacts with, and is phosphorylated by, the kinase domain of SRK in vitro. KAPP was also shown to dephosphorylate SRK. These results, together with the observation that SRK is phosphorylated in vivo approximately 1 h after an incompatible pollination (Cabrillac et al., 2001), suggest that KAPP may play a role in SRK down-regulation, as has been proposed for CLV1 and FLS2 (Williams et al., 1997; Stone et al., 1998; Gomez-Gomez et al., 2001). KAPP may not be the only protein phosphatase involved in the SI response because a breakdown of SI has been observed in pistils after treatment with okadaic acid or microcystin LR (Rundle et al., 1993; Scutt et al., 1993). Note, however, that okadaic acid and microcystin LR are inhibitors of type 1 and type 2A phosphatases and would not be expected to inhibit type 2C phosphatases such as KAPP.
Four previously unidentified interacting proteins were recovered by a two-hybrid screen carried out to search for new SRK-interacting proteins. One of these interactors, calmodulin, was of interest because it is known to be a component of many signal transduction pathways in both plants and animals (Sanders et al., 1999; Chin and Means, 2000). Calmodulin has been shown to interact with two animal receptor kinases: the epidermal growth factor receptor (EGFR) and the insulin receptor (Graves et al., 1985; San José et al., 1992; Joyal et al., 1996; Martín-Nieto and Villalobo, 1998; Feinmesser et al., 1999). However, the role of these interactions is not yet clear. Autophosphorylation of purified rat (Rattus norvegius) liver EGFR was inhibited by calmodulin in vitro, suggesting a direct role for calmodulin in down-regulation of the EGFR (San José et al., 1992). In contrast, the kinase activity of human EGFR, overexpressed in murine fibroblasts, was not inhibited by calmodulin binding (Martín-Nieto and Villalobo, 1998). However, binding of calmodulin may also have more subtle effects because it is known to regulate intracellular trafficking of EGFR (Tebar et al., 2002), and this regulation is thought to be mediated, at least in part, by its antagonistic effect on phosphorylation of EGFR by protein kinase C.
Calcium fluxes have been measured in both the cytoplasm and in the cell wall of stigmatic papillar cells after both compatible and incompatible pollination (Dearnaley et al., 1997; Elleman and Dickinson, 1999). These fluxes are associated with the region of the cell that contacts the pollen grain, and small, localized peaks in cytoplasmic calcium concentration have been correlated with subsequent hydration of pollen grains in B. napus (Dearnaley et al., 1997; Goring, 2000). Therefore, a possible role of calmodulin in vivo might be to mediate cross talk between calcium signaling occurring during pollination and SI signal transduction.
In addition to SRK, calmodulin interacted in a calcium-dependent manner with the kinase domains of three distantly related PRKs (SFR1, RLK4, and CLV1) but not with the kinase domain of BRI1. This suggests that calmodulin may interact with a broad range of PRKs.
A short region from within sub-domain VIa of the SRK kinase domain resembled a calmodulin-binding site based on its predicted amphiphilic α-helical structure, and this region was shown to bind calmodulin when fused to the C terminus of GST. Amphiphilic helices are predicted to occur in the subdomain VIa regions of SFR1, RLK4, and CLV1 but not BRI1, indicating a correlation between the presence of an amphiphilic helix and the ability to bind calmodulin. Sub-domain VIa is located close to the predicted ATP-binding site and the activation loop of the kinase domain, and this region is thought to play a structural role in PRKs (Torii and Clark, 2000). Interestingly, in the Arabidopsis Landsberg erecta mutant, a highly conserved hydrophobic residue (Ile-750) in sub-domain VIa of the ERECTA receptor kinase is replaced by a positively charged Lys residue (Torii et al., 1996). Secondary structure predictions indicate that ERECTA, like SRK, possesses an amphiphilic helix in this region of the kinase domain (even if the helix is less clearly predicted than that of SRK) and the replacement of Ile-750 by a Lys is predicted to modify the amphiphilicity of this putative helix. It would be interesting to determine whether this mutation affects binding to calmodulin.
The interaction observed between SRK and Brassica SNX1 is of particular interest because sorting nexins have been implicated in the down-regulation of animal receptor kinases. Several members of the sorting nexin family have been shown to interact with RTKs or RSKs both in vitro and in vivo (Kurten et al., 1996; Haft et al., 1998; Chin et al., 2001; Parks et al., 2001; Phillips et al., 2001) and modification of the rates of internalization and degradation of the EGFR and the platelet-derived growth factor receptor have been observed after overexpression of sorting nexins (Kurten et al., 1996; Phillips et al., 2001).
In yeast, the SNX1 homolog Vps5p/Grd2p is part of the retromer complex that is essential for retrograde transport from the endosome to the Golgi (Seaman et al., 1998). In addition to Vps5p, the retromer complex includes Vps26p, Vps29p, Vps35p, and Vps17p, and human orthologs of the first three of these proteins have been shown to form a complex that includes SNX1 (Haft et al., 2000). Moreover, human SNX1 reversibly associates with a specific endosomal compartment, again indicating a role in protein trafficking (Kurten et al., 1996, 2001; Teasdale et al., 2001).
Apart from the PX domain, no other sequences are widely conserved in sorting nexins (Teasdale et al., 2001). It is, therefore, significant that the similarity between the Brassica and human SNX1 proteins extends outside the PX domain, indicating that the two proteins carry out a similar function. Interestingly, the Arabidopsis genome is also predicted to encode orthologs of the retromer complex proteins Vps26p (accession no. Q9T091), Vps29p (CAB41864), and Vps35p (NM_127333). Plants, therefore, potentially possess all the components of a sorting nexin-based, vesicle trafficking system. It is possible that KAPP, which plays an important role in the internalization of AtSERK1 (Shah et al., 2002), interacts functionally with such a system to mediate PRK endocytosis.
In contrast to previous studies, which have indicated that plant and animal receptor kinases interact with structurally distinct sets of cytoplasmic proteins (Bower et al., 1996; Gu et al., 1998; Stone et al., 1998; Trotochaud et al., 1999; Schlessinger, 2000), we show here that SRK interacts with two proteins, calmodulin and SNX1, that are closely homologous to animal receptor kinase interactors. It has been proposed that the evolution of membrane-spanning receptor kinases occurred independently in plants and animals, perhaps coinciding with the evolution of multicellularity (Shiu and Bleecker, 2001; Cock et al., 2002). If this was the case, then the interactions with calmodulin and SNX1 in plants and animals were either acquired by convergent evolution, or these proteins already interacted with the common ancestor of plant and animal receptor kinases, which is thought to have been cytosolic (Shiu and Bleecker, 2001). Further analysis of the function of calmodulin and SNX1 in the regulation of receptor kinases in these two kingdoms should help distinguish between these two possibilities.
MATERIALS AND METHODS
Cloning the Brassica oleracea Homolog of KAPP
An internal fragment of the Brassica KAPP coding sequence was amplified from a stigma cDNA library in the yeast (Saccharomyces cerevisiae) two-hybrid vector, pAD-Gal4 2.1 (see below), using two oligonucleotides, Kap1 (5′-GGAGGGATCCCAAGTTGGCTGTTCCTGGAAGTCAT-3′) and Kap2 (5′-CGCTGGATCCGGAAGTTTCCTGCCTCCTCGACGCAT-3′), based on the Arabidopsis KAPP gene sequence. Overlapping fragments corresponding to the 5′ and 3′ ends of the KAPP cDNA were then amplified using the two above oligonucleotides and two additional Brassica KAPP oligonucleotides, Kap3 (5′-TGGGCTTCAATTTGCTGTTCACTCC-3′) and Kap4 (5′-GGGATCTGAGGCCACACCAACT-3′), in combination with vector oligonucleotides from a second stigma cDNA library (Giranton et al., 1995) using a nested PCR amplification approach. Multiple cloned PCR fragments were sequenced to obtain the final definitive cDNA sequence.
cDNA Library Construction and Yeast Two-Hybrid Experiments
Total RNA was extracted, using the method described by Cock et al. (1997), from 600 stigmas of a B. oleracea line homozygous for the S3 SI haplotype (Delorme et al., 1995). A cDNA library was constructed from poly(A+) RNA in the HybriZAP vector (Stratagene, La Jolla, CA). Gene fusions of KAPP and ARC1 sequences with the Gal4 activation domain (Gal4AD) were constructed in pACT2 (CLONTECH, Palo Alto, CA). All gene fusions with the Gal4 DNA-binding domain (Gal4DB), including the kinase domains of SRK29 (amino acids 469–854; accession nos. T14519, 30) and SFR1 (amino acids 469–850; accession nos. T14519, 31), the KI domain of Brassica KAPP (amino acids 110–314), and the C-terminal domain of Brassica ARC1 (amino acids 36–661), were constructed in pAS2 (CLONTECH). Mutant kinase domains lacking kinase activity (SRK29K561R and SFR1K555R) were prepared with the Quickchange express kit (Stratagene).
For two-hybrid assays, yeast strains PJ69-4A (James et al., 1996) and Y190 (Flick and Johnston, 1990) were cotransformed using a lithium acetate-based protocol (Gietz et al., 1992) and grown on standard media (Anonymous, 2001).
Protein Expression and Purification
Gene fusions of the wild-type and mutant kinase domains of SRK29 and SFR1 with GST were constructed (Mazzurco et al., 2001; this work) in pGEX vectors (Pharmacia Corporation, North Peapack, NJ). The kinase domains of RLK4, BRI1, CLV1, and a kinase-negative form of CLV1 were expressed in Escherichia coli as fusion proteins with either MBP (for RLK4 and CLV1) or with GST (for BRI1). The fusion protein GST::KAPP included full-length Brassica KAPP without its signal peptide. GST::HEL1 included a fragment of sub-domain VIa of the SRK3 kinase domain (codons 617–643). A similar fusion protein carrying a non-helical, nonamphiphilic peptide of the same size and of similar hydrophilicity was called GST::PEP1. Expression of GST fusion proteins in E. coli and purification on a glutathione-Sepharose column were as described (Mazzurco et al., 2001), except for GST::SRK29K561R, which was grown at 20°C. Hexa-His-tagged Brassica SNX1 and its deleted form (codons 1–157 including the PX domain) were constructed in pQE32 (Qiagen S.A., Courtaboeuf, France) and expressed as described (Channelière et al., 2002).
Recombinant baculovirus containing the hexa-His-tagged Brassica calmodulin 2 was obtained with the Bac-to-Bac baculovirus expression system (Invitrogen, Carlsbad, CA). Hexa-His-tagged wild-type and kinase-negative forms of the mSRK3 (SRK3His and SRK3His) and Brassica calmodulin 2 were expressed in insect cells as described (Giranton et al., 2000). Calmodulin 2 was purified on nickel-nitrilotriacetic acid agarose beads (Qiagen S.A.).
Binding Assays
Purified GST::kinase fusion proteins (2 μg in 300 μL) were incubated in 50 mm HEPES (pH 7.4), 150 mm NaCl, 1 mm dithiothreitol (DTT), 5 mm CaCl2, and antiprotease cocktail (Boehringer Ingelheim GmbH, Ingelheim am Rhein, Germany) with 100 μL of calmodulin-agarose resin (Sigma, St. Louis) for 1 h at 4°C in Eppendorf tubes with gentle agitation. The resin was washed with 40 column volumes of the binding buffer, and proteins eluted in 50 mm HEPES (pH 7.4), 150 mm NaCl, 1 mm DTT, 5 mm EGTA, and antiprotease cocktail were detected by immunoblotting using an anti-GST antibody (Pharmacia Corporation). Before carrying out the calmodulin-binding assay, mSRK3His expressed in insect cells was solubilized in 62.5 mm Tris-HCl (pH 6.8), 2.5% (w/v) SDS, 0.13 m DTT, and 20% (w/v) glycerol by heating at 100°C for 10 min and desalted twice on Bio-Gel P6-DG columns (Bio-Rad, Hercules, CA).
For kinase domain affinity-binding assays, 1 μg of GST::SRK29kin or GST::SRK29kinK561R was bound to glutathione-Sepharose beads (100 μL) and then incubated with 2 μg of Brassica calmodulin. Washing and elution were as above, and proteins were detected on silver-stained SDS-PAGE gels.
Binding to SNX1 was detected by incubating E. coli extracts containing equivalent amounts of soluble, recombinant GST- or MBP-kinase domain fusion proteins in affinity lysis buffer (50 mm HEPES [pH 7.4], 150 mm NaCl, 10 mm EDTA, 1 mm DTT, and antiprotease cocktail) with 25 μL of glutathione-Sepharose (Amersham Pharmacia Biotech Inc., Piscataway, NJ) or amylose-Sepharose resin (New England BioLabs, Beverly, MA), respectively. The resin was washed once with affinity wash buffer (50 mm HEPES [pH 7.4], 150 mm NaCl, 10 mm EDTA, and 1 mm DTT) and then incubated with E. coli extracts containing recombinant hexa-His-nexin fusion proteins in affinity lysis buffer. After washing with 120 column volumes of affinity wash buffer, fusion proteins and their interacting partners were eluted twice with 25 μL of either 10 mm glutathione, 10 mm Tris (pH 8), or affinity wash buffer plus 10 mm maltose. All experiments were repeated at least once.
Phosphorylation and Dephosphorylation Assays
Phosphorylation assays were carried out using either the procedure described by Williams et al. (1997) or, when using microsomes, that of Cabrillac et al. (2001). KAPP phosphatase activity was detected as described by Williams et al. (1997) using radiolabeled, autophosphorylated GST::SRK29kin prepared using the procedure described by Giranton et al. (2000).
Sequence Analysis and Secondary Structure Predictions
Sequence data analysis and construction of multiple sequence alignments were carried out using Lasergene sequence analysis software (DNASTAR, London). Neighbor joining trees were constructed from multiple alignments using ClustalW (Thompson et al., 1994) and NJplot (Perrière and Gouy, 1996). Secondary structure was predicted using a panel of programs available at the Web site of the Pôle Bio-Informatique Lyonnais (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sspred.html).
Reverse Transcriptase (RT)-PCR and RNA Gel-Blot Analyses
Total RNA was isolated from a range of different organs of B. oleracea line P57Si using the method described by Cock et al. (1997). RT-PCR and RNA gel blot experiments were carried out as described by Channelière et al. (2002).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Shahinez Madi, Claire Rollin, Anne-Marie Thierry, Hervé Leyral, and Claudia Bardoux for technical assistance; Masao Watanabe for the SRK29 cDNA clone; and Daphne Goring, Erin Morris, John Walker, Steve Clark, and Steve Clouse for various PRK domain expression constructs. We are grateful to Gwyneth Ingram for her comments on the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.023846.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Anonymous (2001) Yeast Protocols Hand Book. CLONTECH Laboratories Inc., Palo Alto, CA
- Bower MS, Matias DD, Fernandes-Carvalho E, Mazzurco M, Gu T, Rothstein SJ, Goring DR (1996) Two members of the thioredoxin-h family interact with the kinase domain of a Brassica S-locus receptor kinase. Plant Cell 8: 1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DM, Stone JM, Walker JC (1997) Interaction of the maize and Arabidopsis kinase interaction domain with a subset of receptor-like protein kinases: implications for transmembrane signaling in plants. Plant J 12: 83–95 [DOI] [PubMed] [Google Scholar]
- Cabrillac D, Cock JM, Dumas C, Gaude T (2001) The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 410: 220–223 [DOI] [PubMed] [Google Scholar]
- Carraway KL III, Sweeney C (2001) Localisation and modulation of ErbB receptor tyrosine kinases. Curr Opin Cell Biol 13: 125–130 [DOI] [PubMed] [Google Scholar]
- Channelière S, Rivière S, Scalliet G, Szecsi J, Jullien F, Dolle C, Vergne P, Dumas C, Bendahmane M, Hugueney P et al. (2002) Analysis of gene expression in rose petals using expressed sequence tags. FEBS Lett 515: 35–38 [DOI] [PubMed] [Google Scholar]
- Chin D, Means AR (2000) Calmodulin: a prototypical calcium sensor. Trends Cell Bio 10: 322–328 [DOI] [PubMed] [Google Scholar]
- Chin LS, Raynor MC, Wei X, Chen HQ, Li L (2001) Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J Biol Chem 276: 7069–7078 [DOI] [PubMed] [Google Scholar]
- Cock JM, Swarup R, Dumas C (1997) Natural antisense transcripts of the S locus receptor kinase gene and related sequences in Brassica oleracea. Mol Gen Genet 255: 514–524 [DOI] [PubMed] [Google Scholar]
- Cock JM (2000) A receptor kinase and the self-incompatibility response in Brassica. In M Kreis, JC Walker, eds, Plant Protein Kinases, Advances in Botanical Research, Vol 32. Academic Press, London, pp 270–298 [Google Scholar]
- Cock JM, Vanoosthuyse V, Gaude T (2002) Receptor kinase signalling in plants and animals: distinct molecular systems with mechanistic similarities. Curr Opin Cell Biol 14: 230–236 [DOI] [PubMed] [Google Scholar]
- Dearnaley JDW, Levina NN, Lew RR, Heath IB, Goring DR (1997) Inter-relationships between cytoplasmic Ca2+ peaks, pollen hydration and plasma membrane conductances during compatible and incompatible pollinations of Brassica napus papillae. Plant Cell Physiol 38: 985–999 [DOI] [PubMed] [Google Scholar]
- Delorme V, Giranton J-L, Hatzfeld Y, Friry A, Heizmann P, Ariza MJ, Dumas C, Gaude T, Cock JM (1995) Characterization of the S locus genes, SLG and SRK, of the Brassica S3 haplotype: identification of a membrane-localized protein encoded by the S locus receptor kinase gene Plant J 7: 429–440 [DOI] [PubMed] [Google Scholar]
- Elleman C, Dickinson HG (1999) Commonalities between pollen/stigma and host/pathogen interactions: calcium accumulation during stigmatic penetration by Brassica oleracea pollen. Sex Plant Reprod 12: 194–202 [Google Scholar]
- Emans N, Zimmermann S, Fischer R (2002) Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Viitanen S, DeGrado WF (1987) Recognition and characterization of calmodulin-binding sequences in peptides and proteins. Methods Enzymol 139: 455–478 [DOI] [PubMed] [Google Scholar]
- Feinmesser RL, Wicks SJ, Taverner CJ, Chantry A (1999) Ca2+/calmodulin-dependent kinase II phosphorylates the epidermal growth factor receptor on multiple sites in the cytoplasmic tail and serine 744 within the kinase domain to regulate signal generation. J Biol Chem 274: 16168–16173 [DOI] [PubMed] [Google Scholar]
- Flick JS, Johnston M (1990) Two systems of glucose repression of the Gal1 promoter in Saccharomyces cerevisiae. Mol Cell Biol 10: 4757–4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D, St. Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giranton J-L, Ariza MJ, Dumas C, Cock JM, Gaude T (1995) The S locus receptor kinase encodes a soluble glycoprotein corresponding to the SRK extracellular domain in Brassica oleracea. Plant J 8: 827–834 [DOI] [PubMed] [Google Scholar]
- Giranton J-L, Dumas C, Cock JM, Gaude T (2000) The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta. Proc Natl Acad Sci USA 97: 3759–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gomez L, Bauer Z, Boller T (2001) Both the extracellular leucinerich repeat domain and the kinase activity of FLS2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13: 1155–1163 [PMC free article] [PubMed] [Google Scholar]
- Goring DR (2000) The search for components of the self-incompatibility signaling pathway(s) in Brassica napus. Ann Bot 85: 147–153 [Google Scholar]
- Graves CB, Goewert RR, McDonald JM (1985) The insulin receptor contains a calmodulin-binding domain. Science 230: 827–829 [DOI] [PubMed] [Google Scholar]
- Gu T, Mazzurco M, Sulaman W, Matias DD, Goring DR (1998) Binding of an arm repeat protein to the kinase domain of the S-locus receptor kinase. Proc Natl Acad Sci USA 95: 382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft CR, de la Luz Sierra M, Bafford R, Lesniak MA, Barr VA, Taylor SI (2000) Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol Biol Cell 11: 4105–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft CR, de la Luz Sierra M, Barr VA, Haft DH, Taylor SI (1998) Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol Cell Biol 18: 7278–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241: 42–52 [DOI] [PubMed] [Google Scholar]
- Heldin C-H (1995) Dimerization of cell surface receptors in signal transduction. Cell 80: 213–223 [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144: 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JL, Crimmins DL, Thoma RS, Sacks DB (1996) Identification of insulin-stimulated phosphorylation sites on calmodulin. Biochemistry 35: 6267–6275 [DOI] [PubMed] [Google Scholar]
- Kachroo A, Schopfer CR, Nasrallah ME, Nasrallah JB (2001) Allele-specific receptor-ligand interactions in Brassica self-incompatibility. Science 293: 1824–1826 [DOI] [PubMed] [Google Scholar]
- Kurten RC, Cadena DL, Gill GN (1996) Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 272: 1008–1010 [DOI] [PubMed] [Google Scholar]
- Kurten RC, Eddington AD, Chowdhury P, Smith RD, Davidson AD, Shank BB (2001) Self-assembly and binding of a sorting nexin to sorting endosomes. J Cell Sci 114: 1743–1756 [DOI] [PubMed] [Google Scholar]
- Li J, Smith GP, Walker JC (1999) Kinase interaction domain of kinase-associated protein phosphatase, a phosphoprotein-binding domain. Proc Natl Acad Sci USA 96: 7821–7826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A (1996) Coiled coils: new structures and new functions. Trends Biochem Sci 21: 375–382 [PubMed] [Google Scholar]
- Martín-Nieto J, Villalobo A (1998) The human epidermal growth factor receptor contains a juxtamembrane calmodulin-binding site. Biochemistry 37: 227–236 [DOI] [PubMed] [Google Scholar]
- Mazzurco M, Sulaman W, Elina H, Cock JM, Goring DR (2001) Further analysis of the interactions between the Brassica S receptor kinase and three interacting proteins (ARC1, THL1 and THL2) in the yeast two-hybrid system. Plant Mol Biol 45: 365–376 [DOI] [PubMed] [Google Scholar]
- Östman A, Böhmer F-D (2001) regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol 11: 258–266 [DOI] [PubMed] [Google Scholar]
- Parks WT, Frank DB, Huff C, Renfrew Haft C, Martin J, Meng X, de Caestecker MP, McNally JG, Reddi A, Taylor SI et al. (2001) Sorting nexin 6, a novel SNX, interacts with the transforming growth factor-beta family of receptor serine-threonine kinases. J Biol Chem 276: 19332–19339 [DOI] [PubMed] [Google Scholar]
- Pastuglia M, Swarup R, Rocher A, Saindrenan P, Roby D, Dumas C, Cock JM (2002) Comparison of the expression patterns of two small gene families of S-related receptor-like kinase genes during the defence response in Brassica oleracea and Arabidopsis thaliana. Gene 282: 215–225 [DOI] [PubMed] [Google Scholar]
- Perrière G, Gouy M (1996) WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78: 364–369 [DOI] [PubMed] [Google Scholar]
- Phillips SA, Barr VA, Haft DH, Taylor SI, Haft CR (2001) Identification and characterization of SNX15, a novel sorting nexin involved in protein trafficking. J Biol Chem 276: 5074–5084 [DOI] [PubMed] [Google Scholar]
- Rundle SJ, Nasrallah ME, Nasrallah JB (1993) Effects of inhibitors of protein serine/threonine phosphatases on pollination in Brassica. Plant Physiol 103: 1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF (1999) Communicating with calcium. Plant Cell 11: 691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San José E, Benguria A, Geller P, Villalobo A (1992) Calmodulin inhibits the epidermal growth factor receptor tyrosine kinase. J Biol Chem 267: 15237–15245 [PubMed] [Google Scholar]
- Sato TK, Overduin M, Emr SD (2001) Location, location, location: membrane targeting directed by PX domains. Science 294: 1881–1885 [DOI] [PubMed] [Google Scholar]
- Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103: 211–225 [DOI] [PubMed] [Google Scholar]
- Schopfer CR, Nasrallah ME, Nasrallah JB (1999) The male determinant of self-incompatibility in Brassica. Science 286: 1697–1700 [DOI] [PubMed] [Google Scholar]
- Scutt CP, Fordham-Skelton AP, Croy RRD (1993) Okadaic acid causes breakdown of self-incompatibility in Brassica oleracea: evidence for the involvement of protein phosphatases in the incompatible response. Sex Plant Reprod 6: 282–285 [Google Scholar]
- Seaman MN, McCaffery JM, Emr SD (1998) A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol 142: 665–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Russinova E, Gadella TW Jr, Willemse J, De Vries SC (2002) The Arabidopsis kinase-associated protein phosphatase controls internalization of the somatic embryogenesis receptor kinase 1. Genes Dev 16: 1707–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba H, Takayama S, Iwano M, Shimosato H, Funato M, Nakagawa T, Che FS, Suzuki G, Watanabe M, Hinata K et al. (2001) A pollen coat protein, SP11/SCR, determines the pollen S-specificity in the self-incompatibility of Brassica species. Plant Physiol 125: 2095–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB (2001) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA 98: 10763–10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB (1991) Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci USA 88: 8816–8820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Trotochaud AE, Walker JC, Clark SE (1998) Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiol 117: 1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Anderson EM, Mullen RT, Goring DR (2003) ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self-incompatible Brassica pollen. Plant Cell 15: 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Arnoldo M, Goring DR (1999) A breakdown of Brassica self-incompatibility in ARC1 antisense transgenic plants. Science 286: 1729–1731 [DOI] [PubMed] [Google Scholar]
- Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K (2000) The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403: 913–916 [DOI] [PubMed] [Google Scholar]
- Takayama S, Shiba H, Iwano M, Shimosato H, Che FS, Kai N, Watanabe M, Suzuki G, Hinata K, Isogai A (2000) The pollen determinant of self-incompatibility in Brassica campestris. Proc Natl Acad Sci USA 97: 1920–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Shimosato H, Shiba H, Funato M, Che FS, Watanabe M, Iwano M, Isogai A (2001) Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413: 534–538 [DOI] [PubMed] [Google Scholar]
- Teasdale RD, Loci D, Houghton F, Karlsson L, Gleeson PA (2001) A large family of endosome-localized proteins related to sorting nexin 1. Biochem J 358: 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar F, Villalonga P, Sorkina T, Agell N, Sorkin A, Enrich C (2002) Calmodulin regulates intracellular trafficking of epidermal growth factor receptor and the MAPK signaling pathway. Mol Biol Cell 13: 2057–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y (1996) The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8: 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU, Clark SE (2000) Receptor-like kinases in plant development. In M Kreis, JC Walker eds, Plant Protein Kinases, Advances in Botanical Research, Vol 32. Academic Press, London, pp 225–267 [Google Scholar]
- Trotochaud AE, Hao T, Wu G, Yang Z, Clark SE (1999) The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein. Plant Cell 11: 393–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Song WY, Ruan DL, Sauter M, Ronald PC, Kende H (1999) Expression of a gibberellin-induced leucine-rich repeat receptor-like protein kinase in deepwater rice and its interaction with kinase-associated protein phosphatase. Plant Physiol 120: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK (1996) The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell 86: 435–444 [DOI] [PubMed] [Google Scholar]
- Williams RW, Wilson JM, Meyerowitz EM (1997) A possible role for kinase-associated protein phosphatase in the Arabidopsis CLAVATA1 signaling pathway. Proc Natl Acad Sci USA 94: 10467–10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Seet LF, Hanson B, Hong W (2001) The Phox homology (PX) domain, a new player in phosphoinositide signalling. Biochem J 360: 513–530 [DOI] [PMC free article] [PubMed] [Google Scholar]