Abstract
Tocopherols are amphipathic antioxidants synthesized exclusively by photosynthetic organisms. Tocopherol levels change significantly during plant growth and development and in response to stress, likely as a consequence of the altered expression of pathway-related genes. Homogentisate phytyltransferase (HPT) is a key enzyme limiting tocopherol biosynthesis in unstressed Arabidopsis leaves (E. Collakova, D. DellaPenna [2003] Plant Physiol 131: 632–642). Wild-type and transgenic Arabidopsis plants constitutively overexpressing HPT (35S::HPT1) were subjected to a combination of abiotic stresses for up to 15 d and tocopherol levels, composition, and expression of several tocopherol pathway-related genes were determined. Abiotic stress resulted in an 18- and 8-fold increase in total tocopherol content in wild-type and 35S::HPT1 leaves, respectively, with tocopherol levels in 35S::HPT1 being 2- to 4-fold higher than wild type at all experimental time points. Increased total tocopherol levels correlated with elevated HPT mRNA levels and HPT specific activity in 35S::HPT1 and wild-type leaves, suggesting that HPT activity limits total tocopherol synthesis during abiotic stress. In addition, substrate availability and expression of pathway enzymes before HPT also contribute to increased tocopherol synthesis during stress. The accumulation of high levels of β-, γ-, and δ-tocopherols in stressed tissues suggested that the methylation of phytylquinol and tocopherol intermediates limit α-tocopherol synthesis. Overexpression of γ-tocopherol methyltransferase in the 35S::HPT1 background resulted in nearly complete conversion of γ- and δ-tocopherols to α- and β-tocopherols, respectively, indicating that γ-tocopherol methyltransferase activity limits α-tocopherol synthesis in stressed leaves.
Tocopherols are a group of lipid soluble antioxidants collectively known as vitamin E that are essential components of animal diets. Dietary vitamin E is required for maintaining proper muscular, immune, and neural function and may be involved in reducing the risk of cancer, cardiovascular disease, and cataracts in humans (Pryor, 2000; Brigelius-Flohe et al., 2002). In plants, tocopherols are believed to protect chloroplast membranes from photooxidation and help to provide an optimal environment for the photosynthetic machinery (Fryer, 1992; Munne-Bosch and Alegre, 2002a). Many of the proposed tocopherol functions in animals and plants are related to their antioxidant properties, the most prominent of which is protection of polyunsaturated fatty acids from lipid peroxidation by quenching and scavenging various reactive oxygen species (ROS) including singlet oxygen, superoxide radicals, and alkyl peroxy radicals (Fukuzawa and Gebicky, 1983; Munne-Bosch and Alegre, 2002a).
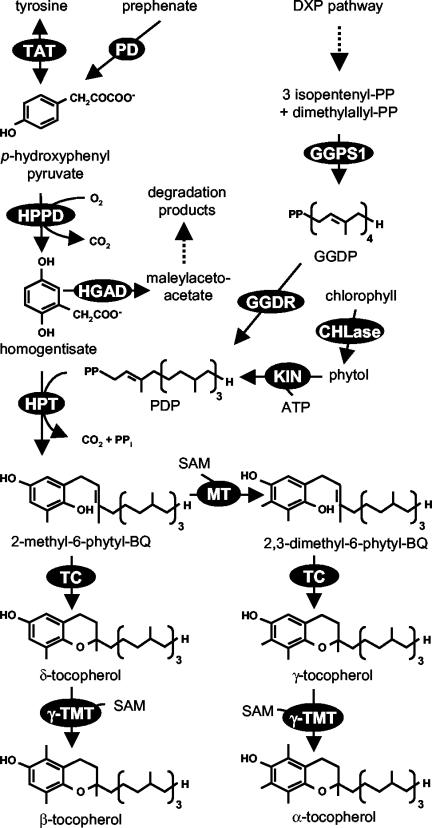
Tocopherols are only synthesized by photosynthetic organisms and consist of a polar chromanol ring and a 15-carbon lipophilic prenyl chain derived from homogentisic acid (HGA) and phytyl diphosphate (PDP; Fig. 1). In plants, HGA is formed from p-hydroxyphenyl pyruvate (HPP) by the cytosolic enzyme HPP dioxygenase (HPPD; Garcia et al., 1997, 1999; Norris et al., 1998). On the basis of radiotracer studies, HPP can originate either from prephenate or Tyr by the shikimate pathway, but the relative contribution of these two precursors to the total HPP pool is unknown (Threlfall and Whistance, 1971; Fiedler et al., 1982; Lopukhina et al., 2001). In plastids, isopentenyl diphosphate derived from the 1-deoxyxylulose-5-phosphate (DXP) pathway (Eisenreich et al., 1998; Lichtenthaler, 1998) is used by geranylgeranyl diphosphate synthase 1 (GGPS1) for the synthesis of geranylgeranyl diphosphate (GGDP; Okada et al., 2000). Three of the four double bonds in the GGDP molecule are reduced to form PDP through partially reduced intermediates by a multifunctional GGDP reductase (GGDR; Addlesee et al., 1996; Keller et al., 1998). Alternatively, PDP can be generated from phytol and ATP by a kinase activity present in chloroplast stroma (Soll et al., 1980).
Figure 1.
Tocopherol biosynthesis in plants. Dashed arrows represent multiple steps. Enzymes are indicated in circles: HPT; TAT; PD, prephenate dehydrogenase; HPPD; HGAD; GGDR; GGPS1; KIN, unspecified kinase; CHLase, chlorophyllase; MPBQ MT; TC; γ-TMT.
Homogentisate phytyltransferase (HPT) is a membrane-bound chloroplast enzyme, which catalyzes the committed step of tocopherol biosynthesis, the condensation of HGA and PDP, to form 2-methyl-6-phytyl-1,4-benzoquinol (MPBQ; Soll,1987; Collakova and DellaPenna, 2001; Savidge et al., 2002). MPBQ can be methylated to 2,3-dimethyl-6-phytyl-1,4-benzoquinol (DMPBQ) by MPBQ methyltransferase (MPBQ MT; Marshall et al., 1985; Soll, 1987; Shintani et al., 2002). MPBQ and DMPBQ can be cyclized by tocopherol cyclase (TC) to form δ- and γ-tocopherol, respectively (Stocker et al., 1996; Arango and Heise, 1998; Porfirova et al., 2002). The last enzyme of the pathway, γ-tocopherol methyltransferase (γ-TMT), catalyzes methylation of γ- and δ-tocopherol to α- and β-tocopherol, respectively (D' Harlingue and Camara, 1985; Shintani and DellaPenna, 1998).
In plants, tocopherol levels and composition vary in different tissues and fluctuate during development and in response to abiotic stresses. Dry and germinating seeds of many plants accumulate predominantly γ-tocopherol, whereas α-tocopherol is the major tocopherol in leaves, which may reflect distinct roles of individual tocopherols in these tissues (Bramley et al., 2000; Franzen and Haas, 1991; Shintani and DellaPenna, 1998). Significant increases in leaf α-tocopherol levels are observed during aging and senescing of plants (Rise et al., 1989; Molina-Torres and Martinez, 1991; Tramontano et al., 1992), possibly to protect cellular components from increased oxidative stress (Munne-Bosch and Alegre, 2002b). Enhanced tocopherol accumulation also occurs in response to a variety of abiotic stresses including high light, drought, salt, and cold and may provide an additional line of protection from oxidative damage (Havaux et al., 2000; Munne-Bosch and Alegre, 2002a).
Although there is a growing body of knowledge about the individual enzymes required for tocopherol biosynthesis in plants, the mechanisms that regulate the overall pathway and result in differential tocopherol content and composition during plant development or stress remain poorly understood. Regulation of tocopherol biosynthesis in senescing and stressed plants may occur at multiple steps of the pathway. HPPD activity limits tocopherol synthesis in non-stressed Arabidopsis plants (Tsegaye et al., 2002), and HPPD mRNA levels are up-regulated in senescing barley (Hordeum vulgare) leaves (Klebler-Janke and Krupinska, 1997). Similarly, various biotic and abiotic stresses elevate Tyr aminotransferase (TAT) mRNA and protein levels and enzyme activity in Arabidopsis (Lopukhina et al., 2001; Sandorf and Hollander-Czytko, 2002). Whether other steps of the tocopherol pathway are also involved in the regulation of tocopherol biosynthesis during stress remains to be determined.
It has been recently demonstrated that HPT activity limits tocopherol synthesis in non-stressed Arabidopsis leaves (Collakova and DellaPenna, 2003). The gene encoding HPT, HPT1, has been cloned from Synechocystis sp. PCC 6803 and Arabidopsis (Collakova and DellaPenna, 2001; Schledz et al., 2001). Overexpression of HPT in Arabidopsis increased leaf and seed tocopherol content by up to 4.4-fold and 75%, respectively (Savidge et al., 2002; Collakova and DellaPenna, 2003). The current study was undertaken to further define the role of HPT in regulating tocopherol biosynthesis in stressed photosynthetic tissues. By combining abiotic stress with molecular and biochemical analyses, we have also identified additional enzymes and/or substrates that limit α-tocopherol synthesis in stressed Arabidopsis leaves.
RESULTS
Biochemical and Physiological Responses of Wild-Type and 35S::HPT1 Plants to Abiotic Stress
Stress is associated with increased total tocopherol levels in a variety of plants (for review, see Munne-Bosch and Alegre, 2002a). We have shown previously that HPT activity is limiting for tocopherol synthesis in non-stressed Arabidopsis leaves (Collakova and DellaPenna, 2003). To investigate whether HPT activity also limits tocopherol synthesis in stressed Arabidopsis leaf tissue, 6-week-old wild type and two well-characterized 35S::HPT1 lines (lines 11 and 54; Collakova and DellaPenna, 2003) were subjected to a combination of nutrient deficiency and high-light stress (0.8–1 mmol photons m–2 s–1) for up to 15 d, and tocopherol content and composition were analyzed during the treatment.
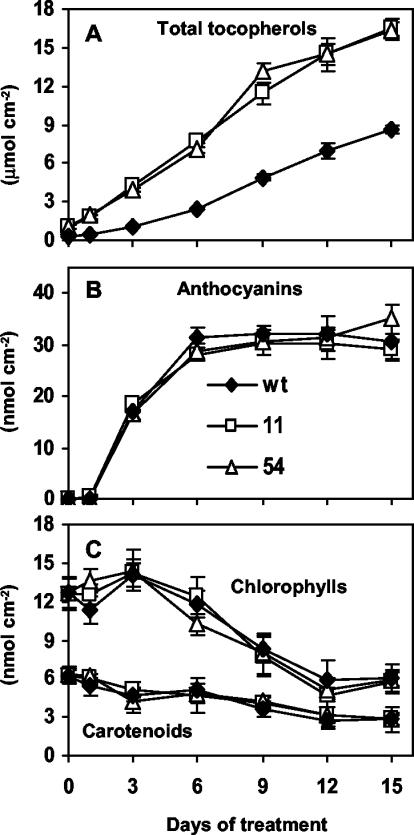
Total tocopherol levels increased in a near linear manner during exposure of both wild-type and 35S::HPT1 plants to stress (R2 ≥ 0.97; Fig. 2A). Before stress treatment, the total tocopherol levels of 6-week-old 35S::HPT1 plants were 3-fold higher than the corresponding wild type (1.06 ± 0.22 and 0.36 ± 0.05 nmol cm–2 leaf area, respectively). In response to 15 d of abiotic stress, total tocopherol levels increased to 16.4 ± 0.8 nmol cm–2 leaf area in 35S::HPT1 and 8.7 ± 0.3 nmol cm–2 leaf area in wild-type plants. In contrast, 15 d of growth in the absence of stress increased tocopherol levels in wild-type and 35S::HPT1 leaves less than 2-fold to 0.49 ± 0.04 and 2.00 ± 0.50 nmol cm–2 leaf area, respectively, most likely as a result of aging. Aging has previously been associated with a moderate increase in leaf tocopherol content in a variety of plants (Rise et al., 1989; Molina-Torres and Martinez, 1991; Tramontano et al., 1992). In 8-week-old plants, the overall increase in total tocopherol levels in stressed relative to non-stressed plants was 18- and 8-fold for wild type and 35S::HPT1, respectively. At any time point during stress treatments, total tocopherol levels in 35S::HPT1 were 1.9- to 3.8-fold higher than wild type (P < 0.006, Fig. 2A), suggesting that HPT activity limits tocopherol synthesis in stressed wild-type Arabidopsis leaves.
Figure 2.
Total tocopherol, anthocyanin, chlorophyll, and carotenoid levels in stressed wild-type and 35S::HPT1 leaves. Plants of the indicated genotypes were grown in a 10-h/14-h light/dark cycle at 75 to 100 μmol photons m–2 s–1 for 6 weeks and then transferred to approximately 900 μmol photons m–2 s–1 growth conditions. A, Total tocopherol levels in leaves of stressed wild-type and 35S::HPT1 plants. High-light stress resulted in a significant elevation of total tocopherol levels in both 35S::HPT1 transgenic and wild-type plants. B, Anthocyanin accumulation in leaves of stressed wild-type and 35S::HPT1 Arabidopsis plants. Anthocyanin levels increased within the first 3 d of stress and reached high steady-state levels after 6 d of stress treatment. C, Chlorophyll and carotenoid degradation in leaves of stressed wild-type and 35S::HPT1 plants. Total chlorophyll and carotenoid levels decreased gradually to approximately 50% of the initial levels in all stressed lines.
The general response to stress was monitored by assessing anthocyanin accumulation and alterations to chlorophyll and carotenoid levels (Fig. 2, B and C). Total anthocyanin levels rapidly increased from below detection to approximately 35 μmol cm–2 leaf area by d 6 and were maintained at this level throughout the stress treatment (Fig. 2B). Total chlorophyll and carotenoid levels decreased gradually during the 15-d stress treatment to approximately half of their initial levels (Fig. 2C). There were no significant differences in chlorophyll, carotenoid, or anthocyanin content between wild type and 35S::HPT1 throughout the course of the experiment (Fig. 2, B and C). It appears that the excess tocopherol accumulated in 35S::HPT1 leaves does not afford additional protection of chlorophylls and carotenoids during stress. Detailed analyses of membrane lipids and their oxidation products under stress conditions are required to more directly address the issue of tocopherol functions in plants.
Other than elevated tocopherol levels, there were no obvious phenotypic differences between wild-type and transgenic plants under normal or stressed conditions. Regardless of genotype, growth of all plants subjected to abiotic stress was inhibited relative to control plants (data not shown), most likely due to a combination of the over-reduced photosystems, oxidative stress, and nutrient deficiency. Because all plants were grown at a 10-h photoperiod, bolting and flowering did not occur during the experimental time frame.
HPT Expression and Enzyme Activity in Unstressed and Stressed Wild Type and 35S::HPT1
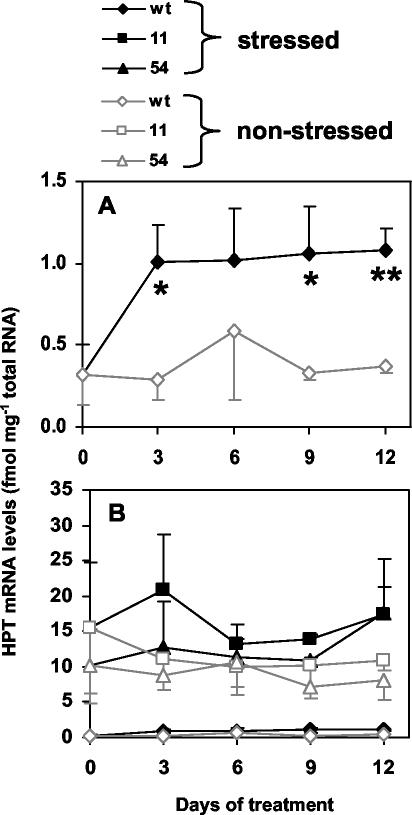
We have shown previously that non-stressed 35S::HPT1 Arabidopsis plants accumulated 20- to 100-fold higher HPT1 mRNA levels than wild type and showed 4- to 10-fold increases in HPT specific activity, which resulted in up to 4.4-fold increased tocopherol levels in leaves of transgenic lines compared with wild type (Collakova and DellaPenna, 2003). In non-stressed wild-type leaves, average HPT mRNA levels ranged from 0.3 to 0.6 fmol mg–1 total RNA during the 12-d experimental time course (Fig. 3A). Consistent with our previous study (Collakova and DellaPenna, 2003), HPT mRNA levels in non-stressed 35S::HPT1 leaves were at least 20-fold higher than wild type and ranged from 7 to 15 fmol mg–1 total RNA during the course of the experiment (Fig. 3B).
Figure 3.
HPT expression in control and stressed wild-type and 35S::HPT1 plants. Plants were grown and stressed as described in Figure 2, total RNA was extracted, and HPT mRNA levels determined by real-time PCR. Data are normalized for EF-1α mRNA levels and presented as average ± sd of three independent experiments. A, Wild-type HPT mRNA levels. B, Wild-type and 35S::HPT1 HPT mRNA levels. Stress resulted in an up-regulation of HPT mRNA levels in wild type, whereas no trend was observed in stressed 35S::HPT1 and the corresponding control plants. * and ** represent P < 0.05 and 0.01, respectively.
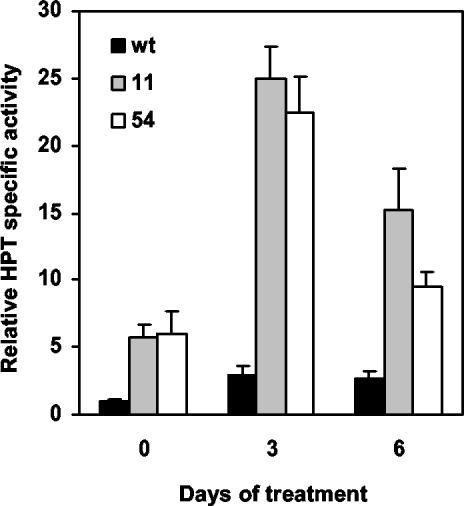
To assess any correlation between elevated total tocopherol levels and changes in HPT expression or activity during abiotic stress, HPT mRNA levels and specific activity were determined in non-stressed and stressed wild-type and 35S::HPT1 plants. HPT mRNA levels were significantly elevated up to 3.5-fold in wild type after 3 d of high-light treatment and remained elevated throughout the course of the experiment (Fig. 3A; P < 0.05). No clear trend was observed for HPT mRNA levels in stressed 35S::HPT1 lines (Fig. 3B), although there was significant biological variation in HPT mRNA levels in both wild-type and 35S::HPT1 plants during stress treatments (Fig. 3, A and B). Consistent with prior studies, HPT specific activity in the absence of abiotic stress was 6-fold higher in 35S::HPT1 lines compared with wild type (Fig. 4; P < 0.0005). In response to 3 and 6 d of stress, HPT specific activity in wild-type and 35S::HPT1 lines increased approximately 3-fold and up to 4.4-fold relative to their respective unstressed controls. In 35S::HPT1, the relative HPT specific activity after 3 d of stress was 9-fold that of comparably treated wild type. After 6 d of stress, HPT specific activity was 5.6- and 3.5-fold higher than the corresponding wild type for 35S::HPT1 lines 11 and 54, respectively (Fig. 4).
Figure 4.
Relative HPT specific activity in control and stressed wild-type and 35S::HPT1 Arabidopsis chloroplasts. Six-week-old plants were transferred to high light (0.8–1 mmol photons m–2 s–1) for 3 and 6 d, and chloroplasts were isolated and assayed for HPT activity. Results from two independent experiments are presented as an average ± sd of the activity increase relative to wild-type non-stressed chloroplasts (0.15 ± 0.10 pmol h–1 mg–1 protein). Abiotic stress resulted in the strong induction of HPT activity relative to control plants in both wild type and 35S::HPT1. Relative HPT specific activity in 35S::HPT1 was significantly higher (P < 0.0005) than in wild type at all time points.
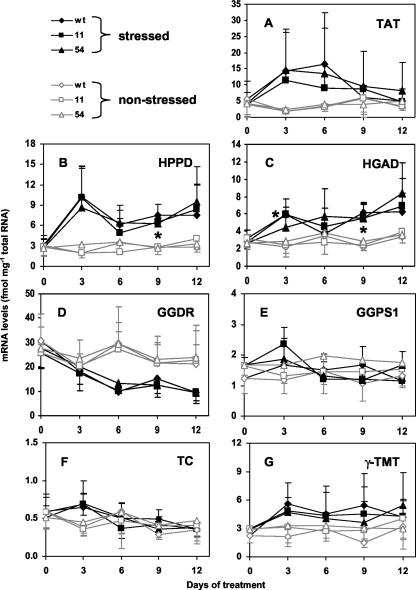
Changes in mRNA Levels of Other Tocopherol Pathway-Related Genes in Non-Stressed and Stressed Wild-Type and 35S::HPT1 Leaves
In addition to HPT, several other tocopherol biosynthetic enzymes (TAT, HPPD, HGAD, GGPS1, GGDR, TC, and γ-TMT) may play roles in regulating tocopherol synthesis in Arabidopsis leaves. Consistent with our previous study (Collakova and Della-Penna, 2003), there were no significant differences between genotypes for mRNA levels of these genes in stressed or unstressed plants (Fig. 5). These results indicate that increased HPT expression in 35S::HPT1 transgenic lines does not significantly impact the expression of other tocopherol pathway-related genes. To assess the role of these enzymes in regulating tocopherol accumulation during stress, their mRNA levels were measured in wild-type and 35S::HPT1 leaves using real-time PCR (Fig. 5). TAT and HPPD catalyze formation of the tocopherol biosynthetic precursors HPP and HGA, respectively, whereas HGAD is involved in HGA degradation (Fig. 1). GGPS1 and GGDR catalyze synthesis of PDP, a prenyl substrate used in tocopherol biosynthesis, whereas TC and γ-TMT are involved in regulating tocopherol composition (Fig. 1). The steady-state mRNA levels of TAT, HPPD, HGAD, GGPS1, and γ-TMT in non-stressed tissues were similar in the different genotypes and varied between 1 and 6 fmol mg–1 total RNA during the 12-d experimental time course (Fig. 5, A, B, C, D, and G). GGDR showed the highest steady-state mRNA levels of all tested genes and fluctuated between 20 and 30 fmol mg–1 total RNA in non-stressed Arabidopsis leaves (Fig. 5E). TC mRNA levels were quite low (0.3–0.6 fmol mg–1 total RNA) and were comparable with wild-type HPT mRNA levels (Figs. 5F and 4A). The overall trends indicated relatively constant steady-state mRNA levels for each gene in the absence of stress. The relatively large ses for some genes in the absence of stress (e.g. GGDR) likely represent the biological variation of the system (Fig. 5).
Figure 5.
Expression of other tocopherol-related genes in control and stressed wild-type and 35S::HPT1 leaves. Experiments were performed as described in Figure 3. A, TAT; B, HPPD; C, HGAD; D, GGDR; E, GGPS1; F, TC; G, γ-TMT. TAT, HPPD, and HGAD mRNA levels increased, whereas GGDR mRNA levels decreased during stress. GGPS1, TC, and γ-TMT mRNA levels were not up-regulated in response to stress. * indicates all three stressed genotypes were statistically different (P < 0.05) from their corresponding unstressed controls at the indicated time points except for HGAD at d 3, where only wild type and 35S::HPT1-11 mRNA levels were statistically different from the corresponding controls.
In response to abiotic stress, TAT, HPPD, and HGAD mRNA levels were elevated severalfold in both wild-type and 35S::HPT1 lines (Fig. 5, A–C), suggesting that these enzymes may also be involved in regulating tocopherol levels during stress. TAT mRNA levels increased 3- to 5-fold relative to non-stressed leaves within the first 6 d of stress before returning near control levels by the end of the time course (Fig. 5A). HPPD and HGAD mRNA levels were elevated throughout the time course of stress treatment (Fig. 5, B and C) and in this regard showed expression profiles similar to HPT mRNA levels in stressed wild type (Fig. 3A). The maximal increase for HGAD and HPPD in stressed relative to non-stressed plants was 2.7- and 5.4-fold, respectively (Fig. 5, B and C). GGPS1, TC, and γ-TMT steady-state mRNA levels were not significantly altered in wild-type or 35S::HPT1 leaves in response to stress (Fig. 5, E–G). The expression profile of GGDR was unique among the genes analyzed in showing a downward trend during stress (Fig. 5D). As with HPT expression during stress, mRNA levels of these other tocopherol pathway-related genes showed high biological variation in response to stress and hence, although clear trends were evident in some expression profiles, most were not statistically different from the corresponding non-stressed controls.
Analysis of Individual Tocopherols and Phytylquinols in Non-Stressed and Stressed Wild-Type and Transgenic Plants
Like most plants, Arabidopsis can synthesize four different tocopherols, α-, β-, γ-, and δ-tocopherols from the phytylquinols MPBQ and DMPBQ by the routes shown in Figure 1. The current study (Figs. 2C, 3, and 4) and previous report (Collakova and Della-Penna, 2003) make it clear that HPT activity is a major limitation in total tocopherol synthesis and accumulation in both non-stressed and stressed leaves. Analyses of tocopherol and phytylquinol levels and compositions were performed to identify steps subsequent to HPT that might also limit α-tocopherol synthesis in non-stressed and stressed leaves of wild-type and 35S::HPT1 Arabidopsis plants. Non-stressed wild-type Arabidopsis leaves accumulated more than 95% α-tocopherol and less than 5% γ-tocopherol (Collakova and DellaPenna, 2003). In non-stressed 35S::HPT1 leaves, α- and γ-tocopherols constituted 90% and 10% of total tocopherol levels, respectively. β-Tocopherol and δ-tocopherol were below detection levels in non-stressed wild-type and 35S::HPT1 Arabidopsis leaves. The phytylquinols MPBQ and DMPBQ were also below detection in non-stressed and stressed wild-type and 35S::HPT1 leaves (data not shown), suggesting that TC activity is not limiting for tocopherol synthesis during normal growth or stress.
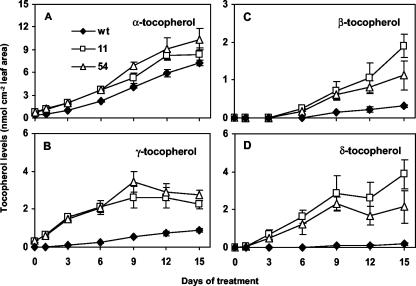
Tocopherol composition changed significantly in both wild-type and 35S::HPT1 leaves during stress. Although α-tocopherol was still the major tocopherol in stressed leaves, β-, γ-, and δ-tocopherols accumulated to high levels as well (Fig. 6). This effect was most pronounced in transgenic leaves after 15 d of stress treatment, where α-tocopherol constituted only 51% and 63% of total tocopherols in 35S::HPT1-11 and -54, respectively, as opposed to 84% in wild type (Fig. 6A). This difference was due to the presence of high levels of other tocopherols in 35S::HPT1 leaves throughout the experimental time course. β- and δ-tocopherols accumulate when MPBQ is directly cyclized to yield δ-tocopherol rather than being methylated to DMPBQ (Fig. 1). Collectively, β- and δ-tocopherols accounted for 0.48 nmol cm–2 leaf area (5.6% of total tocopherols) in stressed wild type and 6 and 3 nmol cm–2 leaf area (35% and 20% of total tocopherols) in stressed 35S::HPT1 lines 11 and 54, respectively (Fig. 6, C and D). Similarly, stressed 35S::HPT1 lines accumulated γ-tocopherol, the immediate precursor of α-tocopherol, to a greater extent than wild type (Fig. 6, B and D). After 15 d of stress, γ-tocopherol constituted 10% of total tocopherols (0.9 nmol cm–2 leaf area) in wild type and 14% and 17% (2.3 and 2.8 nmol cm–2 leaf area) in 35S::HPT1-11 and -54, respectively (Fig. 6, B and D). These results collectively indicate that both the methylation of MPBQ to DMPBQ and γ-tocopherol to α-tocopherol limit α-tocopherol synthesis in stressed 35S::HPT1 leaves.
Figure 6.
Tocopherol levels and composition in stressed wild-type and 35S::HPT1 plants. A, α-Tocopherol; B, γ-tocopherol; C, β-tocopherol; D, δ-tocopherol. Leaves of stressed 35S::HPT1 plants accumulated slightly more α-tocopherol than those of wild-type plants. Large differences are evident in the levels of β-, γ-, and δ-tocopherol between 35S::HPT1 and wild type.
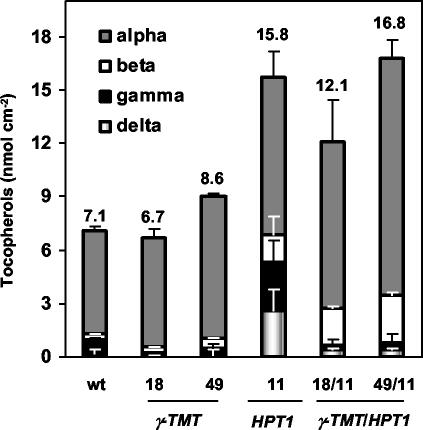
To distinguish between a limitation in γ-TMT activity and S-adenosyl-L-methionine (SAM) levels, 35S::HPT1 lines were crossed to previously characterized lines overexpressing γ-TMT (35S::γ-TMT), which should eliminate any limitation to γ-TMT activity in vivo (Shintani and DellaPenna, 1998; Collakova and DellaPenna, 2003). We have shown previously that under unstressed conditions, excess γ-tocopherol synthesized in 35S::HPT1 was methylated to α-tocopherol in 35S::HPT1/35S::γ-TMT double overexpressers (Collakova and DellaPenna, 2003). During stress, γ-TMT overexpression resulted in reduced γ- and δ-tocopherol levels in 35S::γ-TMT and 35S::HPT1/35S::γ-TMT lines relative to wild type and 35S::HPT1, respectively, without affecting total tocopherol levels (Fig. 7). In stress-treated 35S::HPT1/35S::γ-TMT double overexpressers, the change in tocopherol composition was significant as γ- and δ-tocopherols constituted less than 5% of total tocopherols compared with 34% in stressed 35S::HPT1. This change was less dramatic in 35S::γ-TMT parental lines, where γ- and δ-tocopherols accounted for less than 6% of total tocopherols compared with 14% in wild-type leaves during stress (Fig. 7). These results suggest that γ-TMT activity and not SAM levels limits α- and β-tocopherol synthesis in stressed wild-type and 35S::HPT1 plants.
Figure 7.
Tocopherol levels and composition in stressed wild-type, 35S::HPT1/35S::γ-TMT double overexpressers, and the corresponding transgenic parents. Plants were stressed for 15 d, and individual tocopherols were analyzed by normal phase HPLC. After 15 d of stress, 35S::HPT1 leaves accumulated 2-fold higher total tocopherol levels relative to wild type. The conversion of γ- and δ-tocopherols to α- and β-tocopherols occurred to a much greater extent in 35S::HPT1/35S::γ-TMT than in 35S::HPT1, suggesting that γ-TMT activity limits α- and β-tocopherol synthesis in 35S::HPT1 leaves during abiotic stress. Numbers above the error bars represent total tocopherol levels in each line.
DISCUSSION
Chloroplasts generate increased levels of excited chlorophylls and ROS including singlet oxygen, superoxide anions, and hydroxyl radicals during stress (Fryer, 1992; Mittler, 2002). Plants have developed numerous highly regulated mechanisms for protection from excessive ROS including a variety of different antioxidants and ROS-detoxifying enzymes that are generally up-regulated in their levels and/or activity during stress (Foyer et al., 1994). Tocopherols are thought to represent a key antioxidant protecting polyunsaturated fatty acids from photooxidation by quenching and scavenging ROS and alkyl peroxy radicals generated during photosynthesis (Fryer, 1992). In plants, tocopherol levels can change significantly depending on tissue type, developmental stage, and environmental conditions (Rise et al., 1989; Havaux et al., 2000; Munne-Bosch and Alegre, 2002a). We have used abiotic stress as a means to increase tocopherol synthesis in wild-type and 35S::HPT1 plants to address basic questions about regulation of the pathway in response to stress.
We have shown previously that phytylation of HGA is a limiting step in tocopherol biosynthesis in non-stressed Arabidopsis leaves (Collakova and DellaPenna, 2003). Consistent with this report, the tocopherol level of leaves from unstressed 6-week-old 35S::HPT1 plants was initially severalfold higher than wild type. To determine whether HPT activity is also limiting for tocopherol synthesis under stress conditions, wild-type and 35S::HPT1 plants were subjected to stress and analyzed. By 15 d of light stress, both wild-type and 35S::HPT1 leaves accumulated greatly elevated levels of total tocopherols relative to their respective unstressed controls. The tocopherol level of stressed 35S::HPT1 leaves remained 2- to 4-fold higher than wild type throughout the 15-d stress treatment. Increased tocopherol levels in stressed wild type correlated with significantly elevated HPT mRNA levels and HPT specific activity relative to unstressed controls. In stressed 35S::HPT1 lines, the already high HPT mRNA levels were not significantly elevated in response to stress, but HPT specific activity was, suggesting that translational or posttranslational processes may play an important role in regulating HPT enzyme activity during stress. Whether this is due to activation of existing enzyme or increased levels of HPT protein could not be addressed because HPT antibodies were not available. Collectively, results from molecular and biochemical analyses indicate that, as in unstressed leaves, HPT expression and activity also limits plant tocopherol biosynthesis under conditions of abiotic stress.
Although HPT specific activity is clearly a key factor regulating total tocopherol synthesis during stress, it appears that other factors are also involved. This hypothesis is supported by the observation that HPT specific activity did not correlate with the linear increase in total tocopherol levels at all time points. First, HPT specific activity was similar in wild-type leaves stressed for 3 and 6 d, although total tocopherol levels nearly doubled during this period. Similarly, in 35S::HPT1 lines, HPT specific activity was reduced significantly in 6-d stressed plants relative to those stressed for 3 d, whereas tocopherol levels doubled during this time. Finally, although HPT specific activity increased nearly 3-fold in wild-type leaves stressed for 6 d, it still did not reach the levels of non-stressed 35S::HPT1 plants, yet total tocopherol levels in these stressed wild-type plants were 2.3-fold higher than non-stressed transgenics. These results suggest that one or more HPT-independent limitations that cannot be overcome by HPT1 overexpression alone in non-stressed Arabidopsis plants are alleviated by stress treatment. Increased availability of substrates for tocopherol synthesis as well as up-regulation of tocopherol pathway-related enzymes upstream of HPT tocopherol levels are likely to contribute to the elevated tocopherol levels produced during stress.
HPT catalyzes the committed step of tocopherol synthesis, condensation of two potentially limiting substrates, HGA and PDP. The aromatic precursor HGA is derived from prephenate or Tyr, both products of the shikimate pathway (Threlfall and Whistance, 1971; Fiedler et al., 1982; Lopukhina et al., 2001; Sandorf and Hollander-Czytko, 2002). In plants, the shikimate pathway has been shown to be regulated at the transcriptional level and globally up-regulated during biotic and abiotic stresses (Gorlach et al., 1995; Herrman and Weaver, 1999; Diaz et al., 2001). Thus, the shikimate pathway may provide an increased pool of prephenate that is at least partially used for elevated tocopherol synthesis in stressed plants. In addition, Tyr is released during protein degradation and may also provide an increased pool of HPP available for tocopherol synthesis during stress. Elevated Tyr catabolism in stressed plants is consistent with our findings that TAT, HPPD, and HGAD mRNA levels were all up-regulated during stress in both wild type and 35S::HPT1. These results are also consistent with prior studies showing that TAT expression was up-regulated in Arabidopsis leaves during various biotic and abiotic stresses (Lopukhina et al., 2001; Sandorf and Hollander-Czytko, 2002) and that HPPD mRNA levels were elevated during senescence-induced stress in barley leaves (Klebler-Janke and Krupinska, 1997). Although increases in HPPD mRNA levels are associated with elevated tocopherol levels during stress, HPPD activity alone is probably a minor component regulating tocopherol biosynthetic pathway because constitutive HPPD overexpression alone or in a HPT1 overexpressing background had no effect on total tocopherol levels in stressed Arabidopsis leaves (data not shown).
As with HGA, there are two known metabolic sources of the other HPT substrate, PDP. First, excess PDP required for tocopherol biosynthesis may be synthesized de novo from DXP pathway-derived isopentenyl diphosphate during stress. In plastids, flux through the DXP isoprenoid biosynthetic pathway is regulated at the level of DXP synthase and DXP reductoisomerase activities, which have been shown to limit isoprenoid biosynthesis in non-stressed plants (Estevez et al., 2001; Mahmoud and Croteau, 2001). Enzymes downstream of the DXP pathway, such as GGPS1 and GGDR were not up-regulated at mRNA levels in response to stress, suggesting that they are not major regulators of tocopherol biosynthesis or, if they are, the activities of these two enzymes are translationally or posttranslationally regulated. The second potential source of PDP is phytol, which is derived from chlorophyll degradation, a process involving several steps including the removal of the phytol tail by chlorophyllase (Matile et al., 1999; Tsuchiya et al., 1999). Chlorophyllase gene expression is induced by treatments with stress-associated compounds such as coronatine and methyl jasmonate (Tsuchiya et al., 1999). A correlation between chlorophyll degradation and tocopherol accumulation during leaf senescence has also been reported (Rise et al., 1989). We observed a large decrease in chlorophyll levels in wild type and 35S::HPT1 during stress, which coincided with the rise in tocopherol levels. If one only considers degradation of existing chlorophyll, the maximal pool of phytol released during the 15-d stress treatment would allow for the synthesis of approximately 6 nmol cm–2 leaf area of total tocopherols in wild-type and 35S::HPT1 leaves. Although this possibility is intriguing, it is still not clear what contribution chlorophyll degradation makes to tocopherol biosynthesis during stress because we lack direct evidence for the incorporation of chlorophyll-derived phytol into tocopherols.
The increase in total tocopherol levels was paralleled by a corresponding increase in α-tocopherol levels in response to abiotic stress. However, large increases in the levels of other tocopherols in stressed wild-type and 35S::HPT1 plants suggested that steps downstream of HPT that regulate tocopherol composition, including TC, MPBQ MT, and γ-TMT, may limit α-tocopherol accumulation during stress. The absence of the phytylquinol intermediates MPBQ and DMPBQ suggests that TC activity is sufficient in both stressed and unstressed plants, and the phytylquinol intermediates are rapidly converted to the corresponding tocopherols or are rapidly degraded by unknown mechanisms. The fact that TC mRNA levels are very low and not up-regulated significantly during stress suggests that TC is very active and/or very stable in stressed Arabidopsis leaves. The presence of β- and δ-tocopherols in stressed wild-type and transgenic Arabidopsis leaves suggests that either MPBQ MT activity or SAM limits α-tocopherol synthesis in these plants. This limitation appears to be more severe in 35S::HPT1 leaves, which accumulate β- and δ-tocopherol at levels severalfold higher than wild type at all time points during stress. On the basis of these results, it appears that the majority of excess MPBQ produced by HPT in stressed transgenic plants is not methylated to DMPBQ, but is rather cyclized to form δ-tocopherol.
The final enzyme of the tocopherol pathway is γ-TMT, which uses SAM to generate α- and β-tocopherol from γ- and δ-tocopherol, respectively (D' Harlingue and Camara, 1985; Shintani and DellaPenna, 1998). Relatively high levels of γ- and δ-tocopherols were detected in stressed wild type and much higher levels in 35S::HPT1 leaves, suggesting that γ-TMT activity or SAM is limiting for α-tocopherol synthesis during abiotic stress. To differentiate these possibilities, tocopherol composition and levels were analyzed in stressed transgenic plants overexpressing HPT and γ-TMT in combination (35S::HPT1/35S::γ-TMT). Conversion of nearly the entire pool of γ- and δ-tocopherols to α- and β-tocopherols, respectively, in stressed 35S::HPT1/35S::γ-TMT indicates that γ-TMT activity, and not SAM, limits α-tocopherol biosynthesis during stress. The persistence of small but similar amounts of γ- and δ-tocopherols in stressed leaves of wild type and all transgenic genotypes implies that these tocopherols are either not accessible to γ-TMT or accumulate at these levels for a distinct functional role.
CONCLUSIONS
The current study represents an initial step toward understanding the molecular and metabolic regulation of tocopherol biosynthesis during abiotic stress in plants. We have focused on determining the changes in steady-state tocopherol levels and pool sizes and correlating this with changes in gene expression for various steps of the pathway. Because we cannot yet experimentally address tocopherol turnover, our data do not represent total tocopherol synthesis or fluxes through the pathway. HPT represents a major limitation for total tocopherol synthesis in both non-stressed and stressed wild-type plants. Although stressed leaves showed increased HPT specific activity relative to non-stressed leaves, HPT activity is not the only component that contributes to the elevated total tocopherol levels observed during stress. The differential production and availability of aromatic and prenyl diphosphate precursors in non-stressed and stressed plants also likely plays an important role in regulating tocopherol levels under various growth conditions. Processes such as protein, Tyr, and chlorophyll degradation as well as de novo precursor synthesis may all contribute to the increased demand for substrates used in tocopherol synthesis during abiotic stress. All enzymes downstream of HPT (TC, MPBQ MT, and γ-TMT) have the potential to regulate leaf tocopherol composition. Our results indicate that methylation of both MPBQ and γ-tocopherol, but not the cyclization of tocopherol intermediates, appears to limit the level of α-tocopherol produced in stressed wild-type and 35S::HPT1 Arabidopsis leaves.
MATERIALS AND METHODS
Plant Growth and Abiotic Stress
The following lines were used in this study: wild-type Arabidopsis (ecotype Columbia) and the previously characterized homozygous transgenic lines 35S::γ-TMT-18 and -49 (Shintani and DellaPenna, 1998) and 35S::HPT1-11 and -54, and the corresponding homozygous double overexpressers obtained by crossing these lines (Collakova and DellaPenna, 2003). To obtain a sufficient amount of replicates and tissue for all analyses, seeds were planted 3 to 4 cm apart in a 96-well format trays and grown at a 10-h photoperiod at 75 to 100 μmol photons m–2 s–1 (22°C/19°C day/night cycle) for 6 weeks. Due to the small pot size of this growth format, plants were watered with 0.5× Hoagland solution instead of water once or twice a week to prevent nutrient depletion. At 6 weeks of age, plants were transferred to high light (0.8–1 mmol photons m–2 s–1, 10-h photoperiod) for stress experiments or maintained at 75 to 100 μmol photons m–2 s–1. To exacerbate oxidative stress, nutrient limitation was also imposed on plants grown at high light by watering only with distilled water during the course of stress treatment. At indicated time points, tissue was harvested for HPLC, real-time PCR, or HPT enzyme assays, within 3 to 5 h after the start of the light cycle. To assess the statistical significance of data, Student's t test of a heteroscedastic type with 2-tailed distribution was performed for all experiments.
Prenyllipid and Anthocyanin Analyses
Each genotype was represented by three plants grown under the same conditions, and each plant was analyzed twice per experiment. This experiment was repeated three times with plants grown at different times. Three representative leaf discs (total surface area: 1.507 cm2, 30–35 mg of fresh weight) per plant were harvested and subjected to lipid extraction (Bligh and Dyer, 1959). Using leaf disc area for normalization was found to be more consistent than normalizing to fresh weight. After phase separation, an aliquot of the aqueous phase was acidified with 0.1 n HCl and the amount of anthocyanins present was measured spectrophotometrically at 520 nm as described previously (Merzlyak and Chivkunova, 2000). The organic phase was dried under vacuum, and lipids were dissolved in hexane. An aliquot of the resulting lipid extract was dried, dissolved in 90% (v/v) methanol, and used to determine chlorophyll and carotenoid content by the spectrophotometric method of Lichtenthaler (1987). Tocopherols were analyzed and quantified by normal phase HPLC as described (Collakova and DellaPenna, 2003).
Real-Time PCR
Tissue harvested from three representative plants was ground in liquid nitrogen and total RNA isolated, and real-time PCR was performed as previously described (Collakova and DellaPenna, 2003). The procedure was modified such that 3 μg of total RNA for each sample was reverse transcribed in triplicate. Each reaction was diluted with 2 volumes of water, and cDNA corresponding to 200 ng of total RNA was subjected to real-time PCR using ABI PRISM Sequence Detection System 7000 (Applied Biosystems, Foster City, CA). Standard curves were constructed for each gene and used to determine the absolute mRNA levels. Relative mRNA levels of elongation factor EF-1α were used to normalize each sample. EF-1α mRNA levels varied over a 2.5-fold range during the stress treatment with no apparent trend (data not shown). This experiment was repeated three times with plants grown at different times. Results from these three independent experiments are presented as average ± sd for each line and time point. Taqman probes, primers, and their final optimized concentrations are presented in Table I.
Table I.
Probes, primers, and their final optimal concentrations used in real-time PCR
Each Taqman probe was labeled with 6-fluorescein at the 5′ end and the quencher TAMRA at the 3′ end.
| Enzyme | Primers/ Probes | Sequence (5′ to 3′) | Optimal Concentration |
|---|---|---|---|
| nm | |||
| HPT | Forward | TCTCTAAAAGACTTCTGTTTGCTATTCG | 900 |
| Reverse | AGTCGAGATTTCGGGTTAATGC | 900 | |
| Probe | AAAGCCTCAGGCTGACCCGCAGT | 175 | |
| HPPD | Forward | CAAGGAGTGTGAGGAATTAGGGATT | 300 |
| Reverse | TCGTCGGCCTGTCACCTAGT | 300 | |
| Probe | ATGATCAAGGGACGTTGCTTCAAATCTTCA | 150 | |
| HGAD | Forward | ACCAACCATCGAGGAGGAAA | 900 |
| Reverse | TAAATACAGTCCTTACAGCACCAACTG | 900 | |
| Probe | ATGACAAAATCAAGCAAGGCCACACCA | 175 | |
| TC | Forward | GTATTTGAGCCTCATTGGCAGAT | 900 |
| Reverse | TCACCGCCCCATTCTATCC | 900 | |
| Probe | ATGGCAGGAGGCCTTTCCACAGG | 200 | |
| GGPS1 | Forward | TCCGGTGAGAGTGGTTCGAG | 900 |
| Reverse | CTTGACCCGCCACTAACCC | 900 | |
| Probe | TTGGAGAATTGGCTAAAGCGATAGGAACAGA | 200 | |
| GGDR | Forward | CACTTAGGGAACACCCAACCA | 900 |
| Reverse | ACTTACTATGAGGATTTAGCTGAGATGTATG | 900 | |
| Probe | AGAAATCCGGCGACACATCATCTCCA | 150 | |
| TAT | Forward | AACCGAAAGCCAACGTTTTG | 900 |
| Reverse | TCTTGTAGATGGAGCGGACTAGGT | 600 | |
| Probe | TTCCGAGTCCCGGCTTCCCATG | 200 | |
| g-TMT | Forward | GGCCAAGAGAGCCAATGATC | 900 |
| Reverse | CGCATCCGCAACTTGGA | 900 | |
| Probe | CGGCTGCTCAATCACTCTCTCATAAGGCT | 200 | |
| EF-1α | Forward | CGAACTTCCATAGAGCAATATCGA | 300 |
| Reverse | GCATGGGTGTTGGACAAACTT | 300 | |
| Probe | ACCACGGTCACGCTCGGCCT | 175 |
Prenyltransferase Assays
Homogentisate phytyl transferase assays were performed using [U-14C]HGA and PDP as substrates and crude chloroplast membrane preparations from wild-type and transgenic plants as described previously (Collakova and DellaPenna, 2003). Each stress experiment involved analysis of control (0 d) and stressed (3 and 6 d) wild-type and 35S::HPT1 plants. Chloroplast membrane isolation and HPT activity assays of all plants were performed the same day to ensure equal treatment of all samples in the experiment. Thus, a set of 6-week-old plants grown under non-stress conditions were transferred to high light, and 3 d later, another set of non-stressed plants was transferred to high light. After an additional 3 d, chloroplast membranes were isolated from a set of non-stressed, 3-d-stressed, and 6-d-stressed wild-type and 35S::HPT1 plants. HPT assays with plants stressed for more than 6 d could not be performed because of inconsistent and insufficient chloroplast membrane yields obtained from such plants.
Stressed chloroplasts of all genotypes accumulated large amounts of starch even after 14 h of darkness, which is likely the reason for the poor chloroplast membrane recovery from stressed tissue. We observed a significant decrease in recovery of chloroplast membranes from plants stressed for 3 and 6 d regardless of genotype. However, protein yields per gram of tissue at a given time point and treatment (e.g. 3 d of stress) were similar for all genotypes, and protein to chlorophyll ratios were not significantly different between non-stressed (2.99 ± 0.44) and stressed (3.33 ± 0.28 for T = 3 d and 3.00 ± 0.32 for T = 6 d) plants. HPT specific activity was calculated by subtracting background HPT activity obtained from control reactions containing radiolabeled HGA without exogenously added PDP from the HPT activity obtained when both substrates were present. HPT specific activity is presented as relative HPT specific activity, which is expressed on a protein basis as a -fold difference relative to HPT specific activity of non-stressed wild-type chloroplasts at time zero.
Acknowledgments
We thank the members of the DellaPenna laboratory for reviewing this manuscript and for their helpful comments.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026138.
References
- Addlesee HA, Gibson LCD, Jensen PE, Hunter CN (1996) Cloning, sequencing and functional assignment of the chlorophyll biosynthesis gene, chlP, of Synechocystis sp. PCC 6803. FEBS Lett 389: 126–130 [DOI] [PubMed] [Google Scholar]
- Arango Y, Heise K-P (1998) Tocopherol synthesis from homogentisate in Capsicum anuum L. (yellow pepper) chromoplast membranes: evidence for tocopherol cyclase. Biochem J 336: 531–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bramley PM, Elmadfa I, Kafatos A, Kelly FJ, Manios Y, Roxborough HE, Schuch W, Sheehy PJA, Wagner K-H (2000) Vitamin E. J Sci Food Agric 80: 913–938 [Google Scholar]
- Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg J-M, Azzi A (2002) The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr 76: 703–716 [DOI] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2001) Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 127: 1113–1124 [PMC free article] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2003) Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis thaliana. Plant Physiol 131: 632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D' Harlingue A, Camara B (1985) Plastid enzymes of terpenoid biosynthesis: purification and characterization of a gamma-tocopherol methyltransferase. J Biol Chem 260: 15200–15203 [PubMed] [Google Scholar]
- Diaz J, Bernal A, Pomar F, Merino F (2001) Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L) seedlings in response to copper stress and its relation to lignification. Plant Sci 161: 179–188 [Google Scholar]
- Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A (1998) The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol 5: R221–R233 [DOI] [PubMed] [Google Scholar]
- Estevez JM, Cantero A, Reindl A, Reichler S, Leon P (2001) 1-Deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276: 22901–22909 [DOI] [PubMed] [Google Scholar]
- Fiedler E, Soll J, Schultz G (1982) The formation of homogentisate in the biosynthesis of tocopherol and plastoquinone in spinach chloroplasts. Planta 155: 511–515 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92: 696–717 [Google Scholar]
- Franzen J, Haas MM (1991) Vitamin E content during development of some seedlings. Phytochemistry 30: 2911–2913 [Google Scholar]
- Fryer MJ (1992) The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 15: 381–392 [Google Scholar]
- Fukuzawa K, Gebicky JM (1983) Oxidation of α-tocopherol in micelles and liposomes by the hydroxyl, perhydroxyl, and superoxide free radicals. Arch Biochem Biophys 226: 242–251 [DOI] [PubMed] [Google Scholar]
- Garcia I, Rodgers M, Lenne C, Rolland A, Sailland A, Matringe M (1997) Subcellular localisation and purification of a p-hydroxyphenylpyruvate dioxygenase from cultured carrot cells and characterization of the corresponding cDNA. Biochem J 325: 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I, Rodgers M, Pepin R, Hssich T, Matringe M (1999) Characterization and subcellular compartmentation of recombinant 4-hydroxyphenylpyruvate dioxygenase from Arabidopsis in transgenic tobacco. Plant Physiol 119: 1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach J, Raesecke H-R, Rentsch D, Regenass M, Roy P, Zala M, Keel C, Boller T, Amrhein N, Schmid J (1995) Temporally distinct accumulation of transcripts encoding enzymes of the prechorismate pathway in elicitotreated, cultured tomato cells. Proc Natl Acad Sci USA 92: 3166–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Bonfils J, Lutz C, Niyogi K (2000) Photodamage of the photosynthetic apparatus and its dependence on the leaf development stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol 124: 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrman KM, Weaver LM (1999) The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol 50: 473–503 [DOI] [PubMed] [Google Scholar]
- Keller Y, Bouvier F, D'Harlingue A, Camara B (1998) Metabolic compartmentation of plastid prenyllipid biosynthesis: evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur J Biochem 251: 413–417 [DOI] [PubMed] [Google Scholar]
- Klebler-Janke T, Krupinska K (1997) Isolation of cDNA clones for genes showing enhanced expression in barley leaves during dark-induced senescence as well as during senescence under field conditions. Planta 203: 332–340 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148: 350–382 [Google Scholar]
- Lichtenthaler HK (1998) The plants' 1-deoxy-d-xylulose-5-phosphate pathway for biosynthesis of isoprenoids. Fett 100: 128–138 [Google Scholar]
- Lopukhina A, Dettenberg M, Weiler EW, Hollander-Czytko H (2001) Cloning and characterization of a coronatine-regulated tyrosine aminotransferase from Arabidopsis. Plant Physiol 126: 1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci USA 98: 8915–8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PS, Morris SR, Threlfall DR (1985) Biosynthesis of tocopherols: a re-examination of the biosynthesis and metabolism of 2-demethyl-6-phytyl-1, 4-benzoquinol. Phytochemistry 24: 1705–1711 [Google Scholar]
- Matile P, Hortensteiner S, Thomas H (1999) Chlorophyll degradation. Annu Rev Plant Physiol Plant Mol Biol 50: 67–95 [DOI] [PubMed] [Google Scholar]
- Merzlyak MN, Chivkunova OB (2000) Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J Photochem Photobiol B Biol 55: 155–163 [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Molina-Torres J, Martinez ML (1991) Tocopherols and leaf age in Xanthium strumarium L. New Phytol 118: 95–99 [Google Scholar]
- Munne-Bosch S, Alegre L (2002a) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21: 31–57 [Google Scholar]
- Munne-Bosch S, Alegre L (2002b) Plant aging increases oxidative stress in chloroplasts. Planta 214: 608–615 [DOI] [PubMed] [Google Scholar]
- Norris SR, Shen X, DellaPenna D (1998) Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol 117: 1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Saito T, Nakagawa T, Kawamukai M, Kamiya Y (2000) Five geranylgeranyl diphosphate synthases expressed in different organs are localized into three subcellular compartments in Arabidopsis. Plant Physiol 122: 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfirova S, Bergmuller E, Tropf S, Lemke R, Dormann P (2002) Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA 99: 12495–12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA (2000) Vitamin E and heart disease: basic science to clinical intervention trials. Free Radic Biol Med 28: 141–164 [DOI] [PubMed] [Google Scholar]
- Rise R, Cojocaru M, Gottlieb HE, Goldschmidt E (1989) Accumulation of α-tocopherol in senescing organs as related to chlorophyll degradation. Plant Physiol 89: 1028–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandorf I, Hollander-Czytko H (2002) Jasmonate is involved in the induction of tyrosine aminotransferase and tocopherol biosynthesis in Arabidopsis thaliana. Planta 216: 173–179 [DOI] [PubMed] [Google Scholar]
- Savidge B, Weiss JD, Wong Y-HH, Lassner MW, Mitsky TA, Shewmaker CK, Post-Beittenmiller D, Valentin HE (2002) Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 129: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schledz M, Seidler A, Beyer P, Neuhaus G (2001) A novel phytyltransferase from Synechocystis sp. PCC 6803 involved in tocopherol biosynthesis. FEBS Lett 499: 15–20 [DOI] [PubMed] [Google Scholar]
- Shintani D, DellaPenna D (1998) Elevating the vitamin E content of plants through metabolic engineering. Science 282: 2098–2100 [DOI] [PubMed] [Google Scholar]
- Shintani DK, Cheng Z, DellaPenna D (2002) The role of 2-methyl-6-phytylbenzoquinone methyltransferase in determining tocopherol composition in Synechocystis sp. PCC6803. FEBS Lett 511: 1–5 [DOI] [PubMed] [Google Scholar]
- Soll J (1987) α-Tocopherol and plastoquinone synthesis in chloroplast membranes. Methods Enzymol 148: 383–392 [Google Scholar]
- Soll J, Kemmerling M, Schultz G (1980) Tocopherol and plastoquinone synthesis in spinach chloroplasts subfractions. Arch Biochem Biophys 204: 544–550 [DOI] [PubMed] [Google Scholar]
- Stocker A, Fretz H, Frick H, Ruttimann A, Woggon W-D (1996) The substrate specificity of tocopherol cyclase. Bioorg Med Chem 4: 1129–1134 [DOI] [PubMed] [Google Scholar]
- Threlfall DR, Whistance GR (1971) Biosynthesis of isoprenoid quinones and chromanols. In T Goodwin, ed, Aspects of Terpenoid Chemistry and Biochemistry. Academic Press, Liverpool, UK, pp 357–404
- Tramontano WA, Ganci D, Pennino M, Dierenfeld ES (1992) Age dependent α-tocopherol concentrations in leaves of soybean and pinto beans. Phytochemistry 31: 3349–3351 [Google Scholar]
- Tsegaye Y, Shintani DK, DellaPenna D (2002) Over-expression of the enzyme p-hydroxyphenylpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol Biochem 40: 913–920 [Google Scholar]
- Tsuchiya T, Ohta H, Okawa K, Iwamatsu A, Shimada H, Masuda T, Takamiya K (1999) Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: finding of a lipase motif and the induction by methyl jasmonate. Proc Natl Acad Sci USA 96: 15362–15367 [DOI] [PMC free article] [PubMed] [Google Scholar]