Abstract
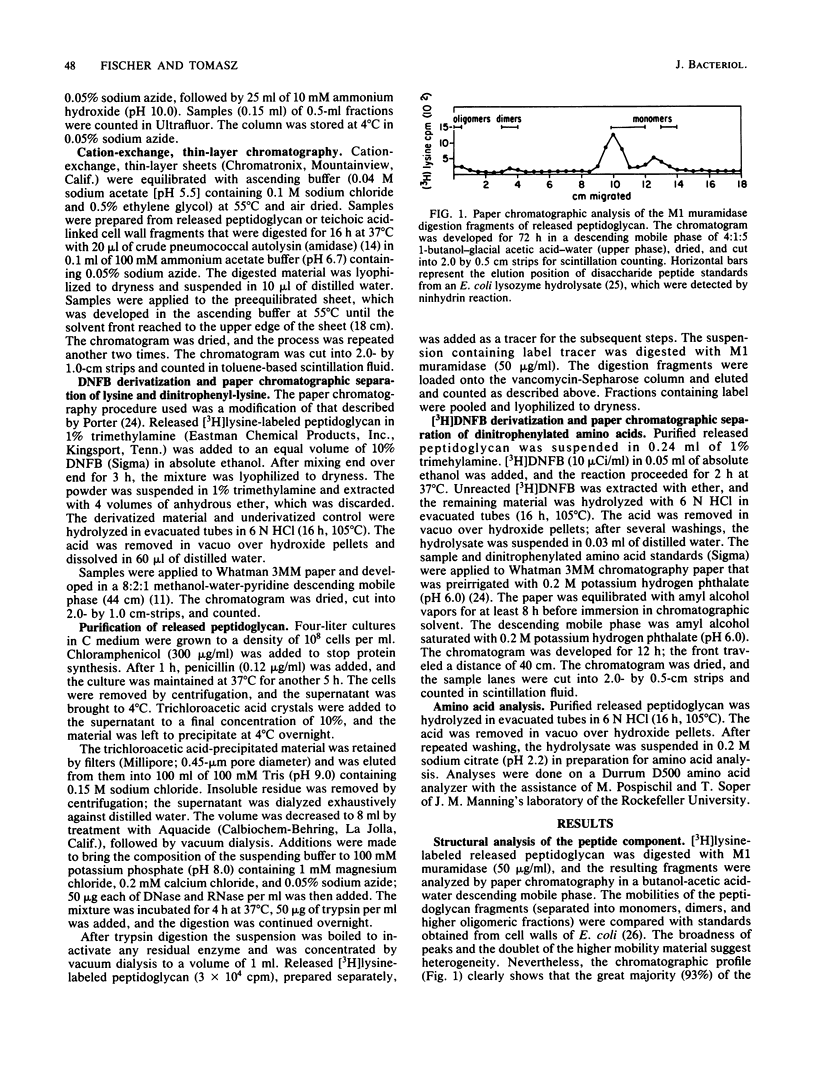
Autolysin-defective pneumococci continue to synthesize both peptidoglycan and teichoic acid polymers (Fischer and Tomasz, J. Bacteriol. 157:507-513, 1984). Most of these peptidoglycan polymers are released into the surrounding medium, and a smaller portion becomes attached to the preexisting cell wall. We report here studies on the degree of cross-linking, teichoic acid substitution, and chemical composition of these peptidoglycan polymers and compare them with normal cell walls. peptidoglycan chains released from the penicillin-treated pneumococci contained no attached teichoic acids. The released peptidoglycan was hydrolyzed by M1 muramidase; over 90% of this material adsorbed to vancomycin-Sepharose and behaved like disaccharide-peptide monomers during chromatography, indicating that the released peptidoglycan contained un-cross-linked stem peptides, most of which carried the carboxy-terminal D-alanyl-D-alanine. The N-terminal residue of the released peptidoglycan was alanine, with only a minor contribution from lysine. In addition to the usual stem peptide components of pneumococcal cell walls (alanine, lysine, and glutamic acid), chemical analysis revealed the presence of significant amounts of serine, aspartate, and glycine and a high amount of alanine and glutamate as well. We suggest that these latter amino acids and the excess alanine and glutamate are present as interpeptide bridges. Heterogeneity of these was suggested by the observation that digestion of the released peptidoglycan with the pneumococcal murein hydrolase (amidase) produced peptides that were resolved by ion-exchange chromatography into two distinct peaks; the more highly mobile of these was enriched with glycine and aspartate. The peptidoglycan chains that became attached to the preexisting cell wall in the presence of penicillin contained fewer peptide cross-links and proportionally fewer attached teichoic acids than did their normal counterparts. The normal cell wall was heavily cross-linked, and the cross-linked peptides were distributed equally between the teichoic acid-linked and teichoic acid-free fragments.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKBURN S., LOWTHER A. G. The separation of N-2:4-dinitrophenly amino-acids on paper chromatograms. Biochem J. 1951 Jan;48(1):126–128. doi: 10.1042/bj0480126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. F., Shockman G. D. Isolation and characterization of soluble peptidoglycan from several strains of Streptococcus faecium. J Bacteriol. 1984 Aug;159(2):511–519. doi: 10.1128/jb.159.2.511-519.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- De Cueninck B. J., Shockman G. D., Swenson R. M. Group B, type III streptococcal cell wall: composition and structural aspects revealed through endo-N-acetylmuramidase-catalyzed hydrolysis. Infect Immun. 1982 Feb;35(2):572–581. doi: 10.1128/iai.35.2.572-581.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Tomasz A. Production and release of peptidoglycan and wall teichoic acid polymers in pneumococci treated with beta-lactam antibiotics. J Bacteriol. 1984 Feb;157(2):507–513. doi: 10.1128/jb.157.2.507-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham W. D., Gilvarg C. Kinetics of cross-linking of peptidoglycan in Bacillus megaterium. J Biol Chem. 1974 Apr 25;249(8):2478–2482. [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y. Structural and immunological studies on the pneumococcal C polysaccharide. J Biol Chem. 1967 Feb 10;242(3):463–470. [PubMed] [Google Scholar]
- Howard L. V., Gooder H. Specificity of the autolysin of Streptococcus (Diplococcus) pneumoniae. J Bacteriol. 1974 Feb;117(2):796–804. doi: 10.1128/jb.117.2.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Purification of the pneumococcal N-acetylmuramyl-L-alanine amidase to biochemical homogeneity. J Biol Chem. 1976 Jul 25;251(14):4199–4207. [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Young N. M. Structure of the complex polysaccharide C-substance from Streptococcus pneumoniae type 1. Biochemistry. 1980 Sep 30;19(20):4712–4719. doi: 10.1021/bi00561a026. [DOI] [PubMed] [Google Scholar]
- Keglević D., Ladesić B., Hadzija O., Tomasić J., Valinger Z., Pokorny M., Naumski R. Isolation and study of the composition of a peptidoglycan complex excreted by the biotin-requiring mutant of Brevibacterium divaricatum NRRL-2311 in the presence of penicillin. Eur J Biochem. 1974 Mar 1;42(2):389–400. doi: 10.1111/j.1432-1033.1974.tb03351.x. [DOI] [PubMed] [Google Scholar]
- LIU T. Y., GOTSCHLICH E. C. The chemical composition of pneumococcal C-polysaccharide. J Biol Chem. 1963 Jun;238:1928–1934. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leon M. A., Young N. M. Specificity for phosphorylcholine of six murine myeloma proteins reactive with Pneumococcus C polysaccharide and beta-lipoprotein. Biochemistry. 1971 Apr 13;10(8):1424–1429. doi: 10.1021/bi00784a024. [DOI] [PubMed] [Google Scholar]
- Manning J. M. Chromatographic determination of the D- and L-amino acid residues in pneumococcal C-polysaccharide. J Biol Chem. 1971 May 10;246(9):2926–2929. [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Poxton I. R., Tarelli E., Baddiley J. The structure of C-polysaccharide from the walls of Streptococcus pneumoniae. Biochem J. 1978 Dec 1;175(3):1033–1042. doi: 10.1042/bj1751033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Barrett J. F. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol. 1983;37:501–527. doi: 10.1146/annurev.mi.37.100183.002441. [DOI] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science. 1967 Aug 11;157(3789):694–697. doi: 10.1126/science.157.3789.694. [DOI] [PubMed] [Google Scholar]
- Tomasz A., McDonnell M., Westphal M., Zanati E. Coordinated incorporation of nascent peptidoglycan and teichoic acid into pneumococcal cell walls and conservation of peptidoglycan during growth. J Biol Chem. 1975 Jan 10;250(1):337–341. [PubMed] [Google Scholar]
- Tynecka Z., Ward J. B. Peptidoglycan synthesis in Bacillus licheniformis. The inhibition of cross-linking by benzylpenicillin and cephaloridine in vivo accompanied by the formation of soluble peptidoglycan. Biochem J. 1975 Jan;146(1):253–267. doi: 10.1042/bj1460253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. The synthesis of peptidoglycan in an autolysin-deficient mutant of Bacillus licheniformis N.C.T.C. 6346 and the effect of beta-lactam antibiotics, bacitracin and vancomycin. Biochem J. 1974 Jul;141(1):227–241. doi: 10.1042/bj1410227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Yu W., Strominger J. L. Linear, uncross-linked peptidoglycan secreted by penicillin-treated Bacillus subtilis. Isolation and characterization as a substrate for penicillin-sensitive D-alanine carboxypeptidases. J Biol Chem. 1980 Dec 10;255(23):11577–11587. [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B. The synthesis of covalently-linked teichoic acid and peptidoglycan by cell-free preparations of Bacillus licheniformis. Biochem Biophys Res Commun. 1975 Aug 4;65(3):877–885. doi: 10.1016/s0006-291x(75)80467-0. [DOI] [PubMed] [Google Scholar]


