Abstract
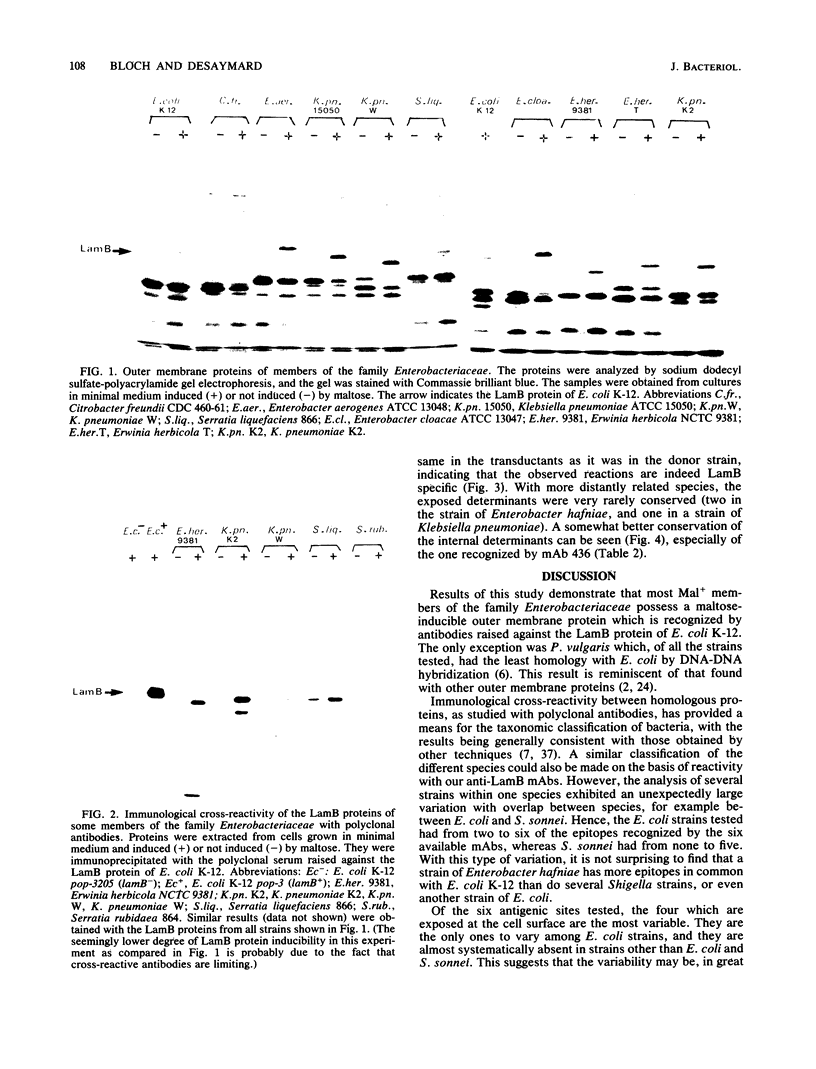
In this study we demonstrate that most members of the family Enterobacteriaceae possess a maltose-inducible outer membrane protein homologous to the LamB protein of Escherichia coli K-12. These proteins react with polyclonal antibodies raised against the LamB protein of E. coli K-12. We compared the antigenic structure of the LamB protein in members of the family Enterobacteriaceae with six monoclonal antibodies raised against the LamB protein of E. coli K-12. Four of them reacted with epitopes located at the outer face of the membrane, and two reacted with epitopes located at the inner face of the membrane. A great degree of variability was observed for the external epitopes. Even in a single species, such as E. coli, an important polymorphism was present. In contrast, the internal epitopes were more conserved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A., Pugsley A. P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the ompA protein of Escherichia coli. J Bacteriol. 1980 Aug;143(2):906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun G., Cole S. T. DNA sequence analysis of the Serratia marcescens ompA gene: implications for the organisation of an enterobacterial outer membrane protein. Mol Gen Genet. 1984;195(1-2):321–328. doi: 10.1007/BF00332766. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Skerman F. J., Falkow S. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J Bacteriol. 1972 Mar;109(3):953–965. doi: 10.1128/jb.109.3.953-965.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J Bacteriol. 1982 May;150(2):722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Clement J. M., Hofnung M. Further sequence analysis of the phage lambda receptor site. Possible implications for the organization of the lamB protein in Escherichia coli K12. J Mol Biol. 1984 May 25;175(3):395–401. doi: 10.1016/0022-2836(84)90355-3. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Lepouce E., Marchal C., Hofnung M. Genetic study of a membrane protein: DNA sequence alterations due to 17 lamB point mutations affecting adsorption of phage lambda. EMBO J. 1983;2(1):77–80. doi: 10.1002/j.1460-2075.1983.tb01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessein A., Schwartz M. Is there a pullulanase in escherichia coli? Eur J Biochem. 1974 Jun 15;45(2):363–366. doi: 10.1111/j.1432-1033.1974.tb03561.x. [DOI] [PubMed] [Google Scholar]
- Elder B. L., Boraker D. K., Fives-Taylor P. M. Whole-bacterial cell enzyme-linked immunosorbent assay for Streptococcus sanguis fimbrial antigens. J Clin Microbiol. 1982 Jul;16(1):141–144. doi: 10.1128/jcm.16.1.141-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Gabay J., Benson S., Schwartz M. Genetic mapping of antigenic determinants on a membrane protein. J Biol Chem. 1983 Feb 25;258(4):2410–2414. [PubMed] [Google Scholar]
- Gabay J., Schwartz M. Monoclonal antibody as a probe for structure and function of an Escherichia coli outer membrane protein. J Biol Chem. 1982 Jun 25;257(12):6627–6630. [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Lipopolysaccharide-deficient, bacteriophage-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):98–108. doi: 10.1128/jb.127.1.98-108.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Hatfield D., Schwartz M. malB region in Escherichia coli K-12: characterization of new mutations. J Bacteriol. 1974 Jan;117(1):40–47. doi: 10.1128/jb.117.1.40-47.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Jezierska A., Braun-Breton C. lamB mutations in E. coli K12: growth of lambda host range mutants and effect of nonsense suppressors. Mol Gen Genet. 1976 May 7;145(2):207–213. doi: 10.1007/BF00269595. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Marchal C., Hofnung M. Negative dominance in gene lamB: random assembly of secreted subunits issued from different polysomes. EMBO J. 1983;2(1):81–86. doi: 10.1002/j.1460-2075.1983.tb01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Pirtle R. M., Inouye M. Homology of the gene coding for outer membrane lipoprotein within various Gram-negative bacteria. J Bacteriol. 1979 Jan;137(1):595–604. doi: 10.1128/jb.137.1.595-604.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J. M. The receptor protein of phage lambda: purification, characterization and preliminary electrical studies in planar lipid bilayers. Ann Microbiol (Paris) 1982 Jan;133A(1):27–32. [PubMed] [Google Scholar]
- Palva E. T. Major outer membrane protein in Salmonella typhimurium induced by maltose. J Bacteriol. 1978 Oct;136(1):286–294. doi: 10.1128/jb.136.1.286-294.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa M., Clément J. M. Location of a phage binding region on an outer membrane protein. FEBS Lett. 1980 Nov 17;121(1):127–129. doi: 10.1016/0014-5793(80)81280-4. [DOI] [PubMed] [Google Scholar]
- Roa M. Interaction of bacteriophage K10 with its receptor, the lamB protein of Escherichia coli. J Bacteriol. 1979 Nov;140(2):680–686. doi: 10.1128/jb.140.2.680-686.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Couture E., Schwartz M. Monoclonal antibodies reveal lamB antigenic determinants on both faces of the Escherichia coli outer membrane. J Bacteriol. 1983 Sep;155(3):1382–1392. doi: 10.1128/jb.155.3.1382-1392.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Tsugita A., Schwartz M., Rosenbusch J. P. Topology of phage lambda receptor protein. Mapping targets of proteolytic cleavage in relation to binding sites for phage or monoclonal antibodies. J Biol Chem. 1984 Jun 25;259(12):7570–7576. [PubMed] [Google Scholar]
- Schwartz M., Le Minor L. Occurrence of the bacteriophage lambda receptor in some enterobacteriaceae. J Virol. 1975 Apr;15(4):679–685. doi: 10.1128/jvi.15.4.679-685.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Phage lambda receptor (lamB protein) in Escherichia coli. Methods Enzymol. 1983;97:100–112. doi: 10.1016/0076-6879(83)97123-9. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Gilberg W. Construction of plasmid vectors with unique PstI cloning sites in a signal sequence coding region. Gene. 1980 Dec;12(3-4):235–241. doi: 10.1016/0378-1119(80)90105-5. [DOI] [PubMed] [Google Scholar]
- Trinel P. A., Kaibous M., Izard D., Gavini F., Leclerc H. Etude immunologique de la glycéraldéhyde-3-phosphate-déshydrogénase chez les Entérobactéries; intérêt taxonomique. Ann Microbiol (Paris) 1983 Mar-Apr;134A(2):127–139. [PubMed] [Google Scholar]
- Wandersman C., Schwartz M., Ferenci T. Escherichia coli mutants impaired in maltodextrin transport. J Bacteriol. 1979 Oct;140(1):1–13. doi: 10.1128/jb.140.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]