Abstract
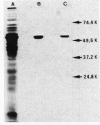
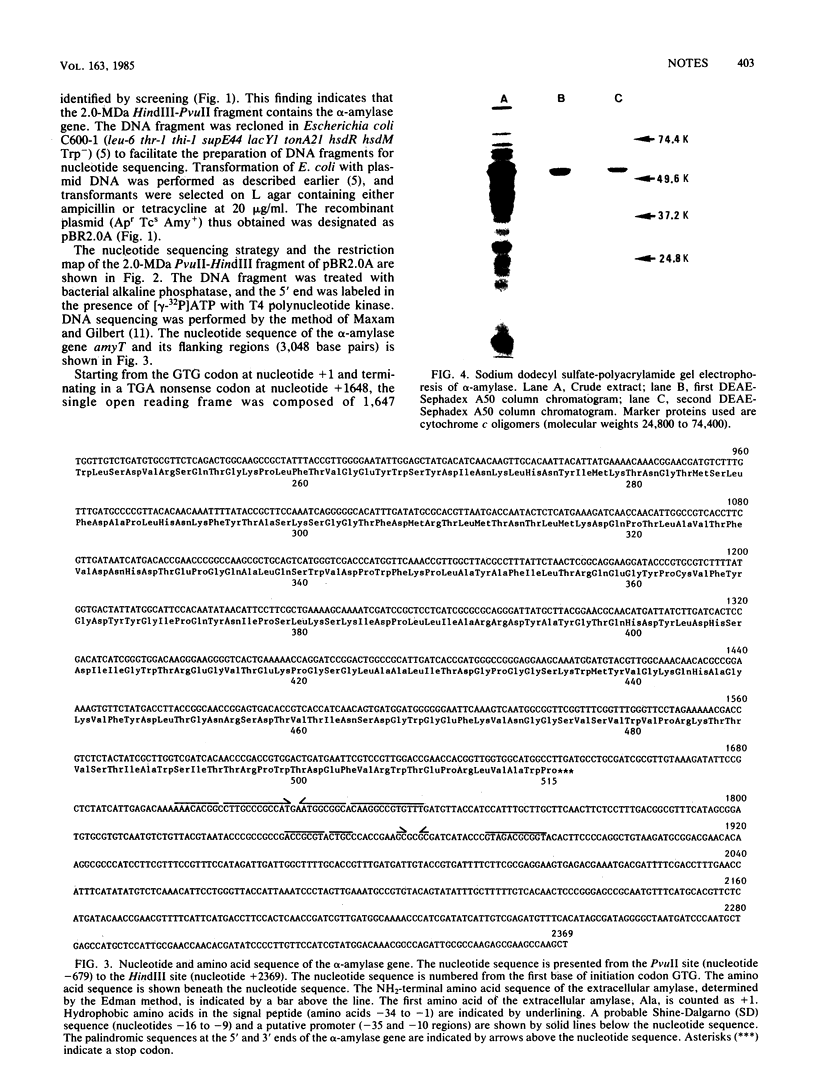
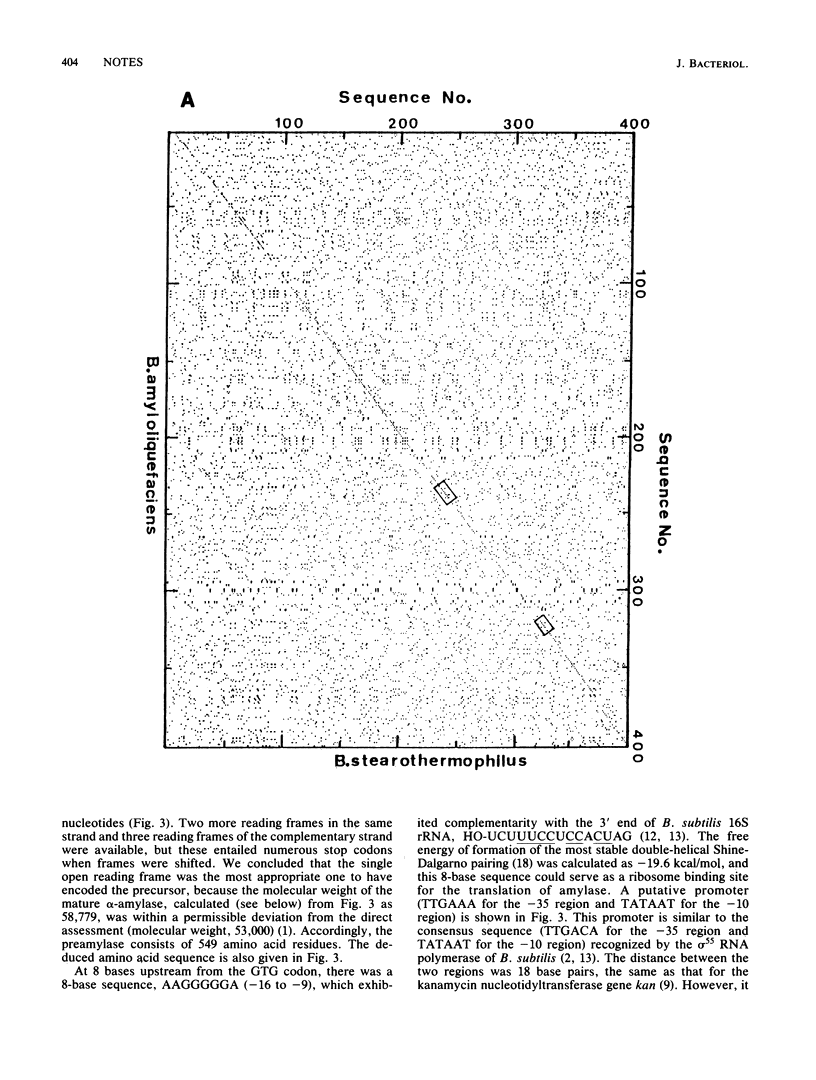
The nucleotide sequence of the Bacillus stearothermophilus alpha-amylase gene and its flanking regions was determined. An open reading frame was found, comprising a total of 1,647 base pairs (549 amino acids) and starting from a GUG codon as methionine. It was shown by NH2-terminal amino acid sequence analysis that the extracellular amylase consisted of 515 amino acid residues, which corresponded to a molecular weight of 58,779. Thus the NH2-terminal portion of the gene encodes 34 amino acid residues as a signal peptide. The amino acid sequence deduced from the alpha-amylase gene was fairly homologous (61%) with that of another thermostable amylase from Bacillus amyloliquefaciens.
Full text
PDF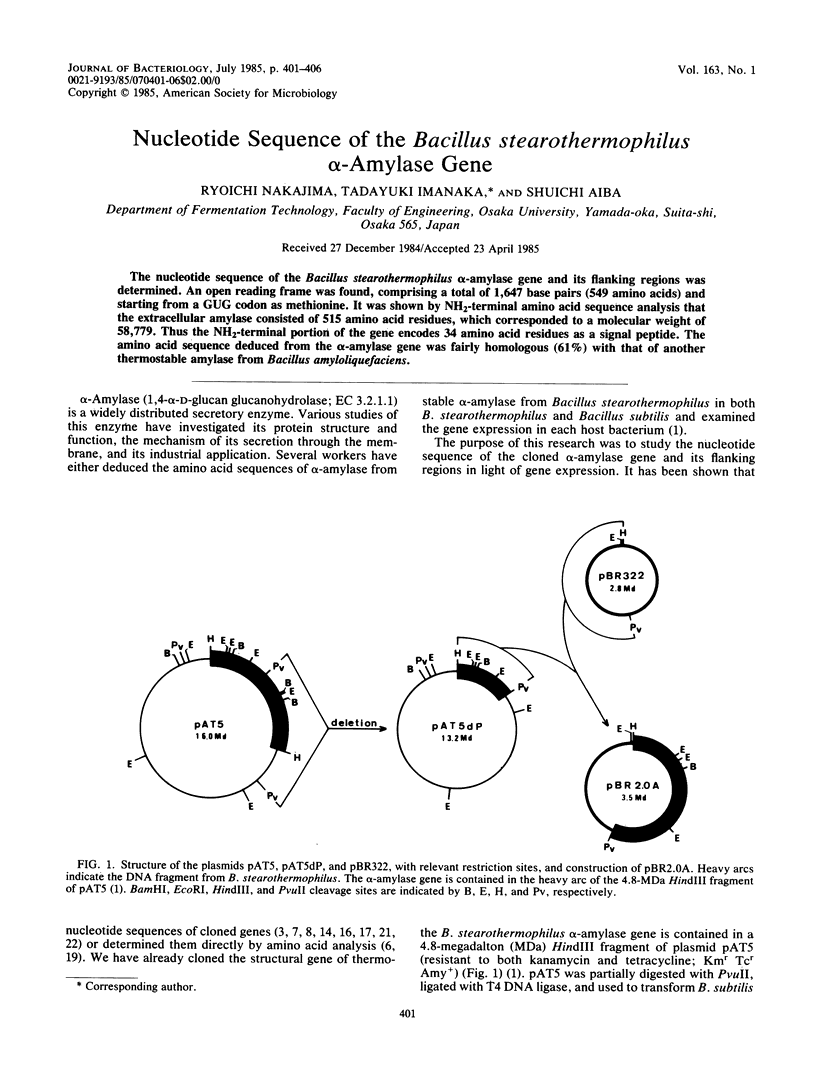


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba S., Kitai K., Imanaka T. Cloning and Expression of Thermostable alpha-Amylase Gene from Bacillus stearothermophilus in Bacillus stearothermophilus and Bacillus subtilis. Appl Environ Microbiol. 1983 Nov;46(5):1059–1065. doi: 10.1128/aem.46.5.1059-1065.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss E. R., Setlow P. Complete nucleotide sequence and start sites for transcription and translation of the Bacillus megaterium protein C gene. J Bacteriol. 1984 Jun;158(3):809–813. doi: 10.1128/jb.158.3.809-813.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aiba S. Isolation and characterization of antibiotic resistance plasmids from thermophilic bacilli and construction of deletion plasmids. J Bacteriol. 1981 Jun;146(3):1091–1097. doi: 10.1128/jb.146.3.1091-1097.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Tanaka T., Tsunekawa H., Aiba S. Cloning of the genes for penicillinase, penP and penI, of Bacillus licheniformis in some vector plasmids and their expression in Escherichia coli, Bacillus subtilis, and Bacillus licheniformis. J Bacteriol. 1981 Sep;147(3):776–786. doi: 10.1128/jb.147.3.776-786.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluh I. Amino acid sequence of hog pancreatic alpha-amylase isoenzyme I. FEBS Lett. 1981 Dec 28;136(2):231–234. doi: 10.1016/0014-5793(81)80624-2. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Crerar M. M., Swain W. F., Pictet R. L., Thomas G., Rutter W. J. Structure of a family of rat amylase genes. Nature. 1980 Sep 11;287(5778):117–122. doi: 10.1038/287117a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Stary S. J., Swift G. H. Two similar but nonallelic rat pancreatic trypsinogens. Nucleotide sequences of the cloned cDNAs. J Biol Chem. 1982 Aug 25;257(16):9724–9732. [PubMed] [Google Scholar]
- Matsumura M., Katakura Y., Imanaka T., Aiba S. Enzymatic and nucleotide sequence studies of a kanamycin-inactivating enzyme encoded by a plasmid from thermophilic bacilli in comparison with that encoded by plasmid pUB110. J Bacteriol. 1984 Oct;160(1):413–420. doi: 10.1128/jb.160.1.413-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H., Yamane K., Yamaguchi K., Nagata Y., Maruo B. Hybrid alpha-amylases produced by transformants of Bacillus subtilis. I. Purification and characterization of extracellular alpha-amylases produced by the parental strains and transformants. Biochim Biophys Acta. 1974 Sep 13;365(1):235–247. doi: 10.1016/0005-2795(74)90268-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Nishide T., Emi M., Nakamura Y., Matsubara K. Corrected sequences of cDNAs for human salivary and pancreatic alpha-amylases [corrected]. Gene. 1984 May;28(2):263–270. doi: 10.1016/0378-1119(84)90265-8. [DOI] [PubMed] [Google Scholar]
- Novotny J. Matrix program to analyze primary structure homology. Nucleic Acids Res. 1982 Jan 11;10(1):127–131. doi: 10.1093/nar/10.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Isolation and sequence analysis of a barley alpha-amylase cDNA clone. J Biol Chem. 1983 Jul 10;258(13):8169–8174. [PubMed] [Google Scholar]
- Takkinen K., Pettersson R. F., Kalkkinen N., Palva I., Söderlund H., Käriäinen L. Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. J Biol Chem. 1983 Jan 25;258(2):1007–1013. [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H., Ohmura K., Nakayama A., Takeichi Y., Otozai K., Yamasaki M., Tamura G., Yamane K. Alpha-amylase genes (amyR2 and amyE+) from an alpha-amylase-hyperproducing Bacillus subtilis strain: molecular cloning and nucleotide sequences. J Bacteriol. 1983 Oct;156(1):327–337. doi: 10.1128/jb.156.1.327-337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Galizzi A., Henner D. Nucleotide sequence of the amylase gene from Bacillus subtilis. Nucleic Acids Res. 1983 Jan 25;11(2):237–249. doi: 10.1093/nar/11.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]