Abstract
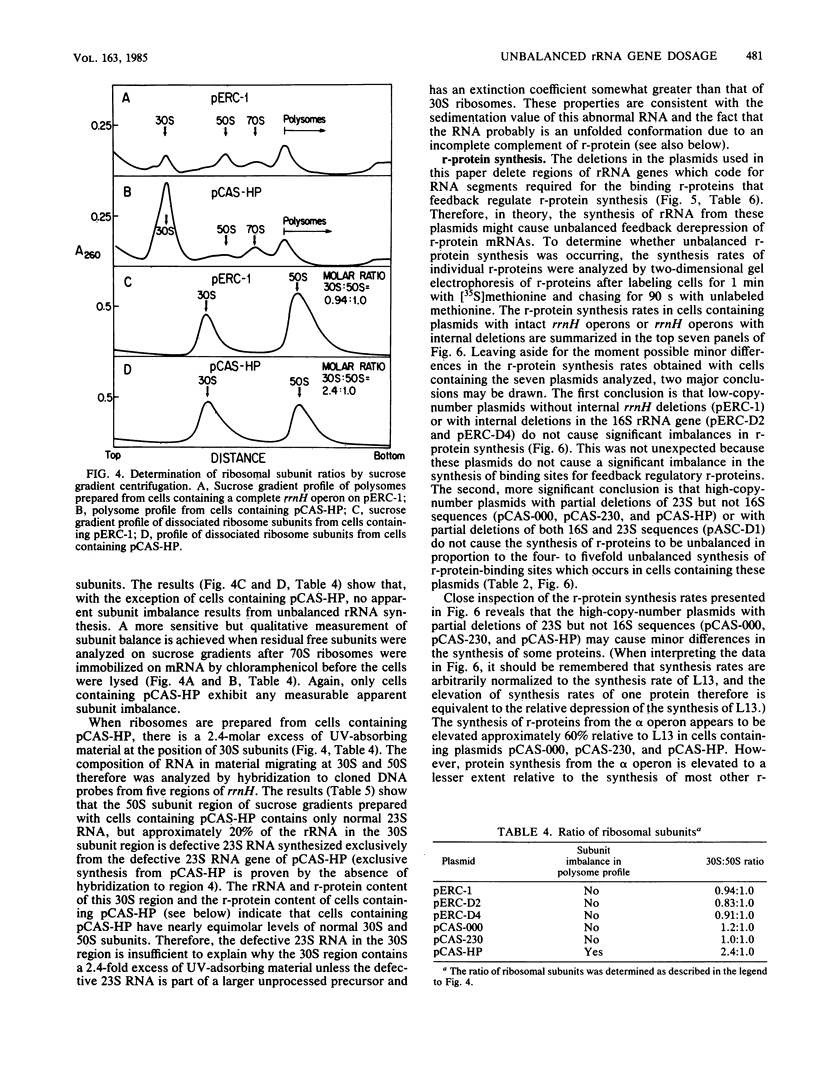
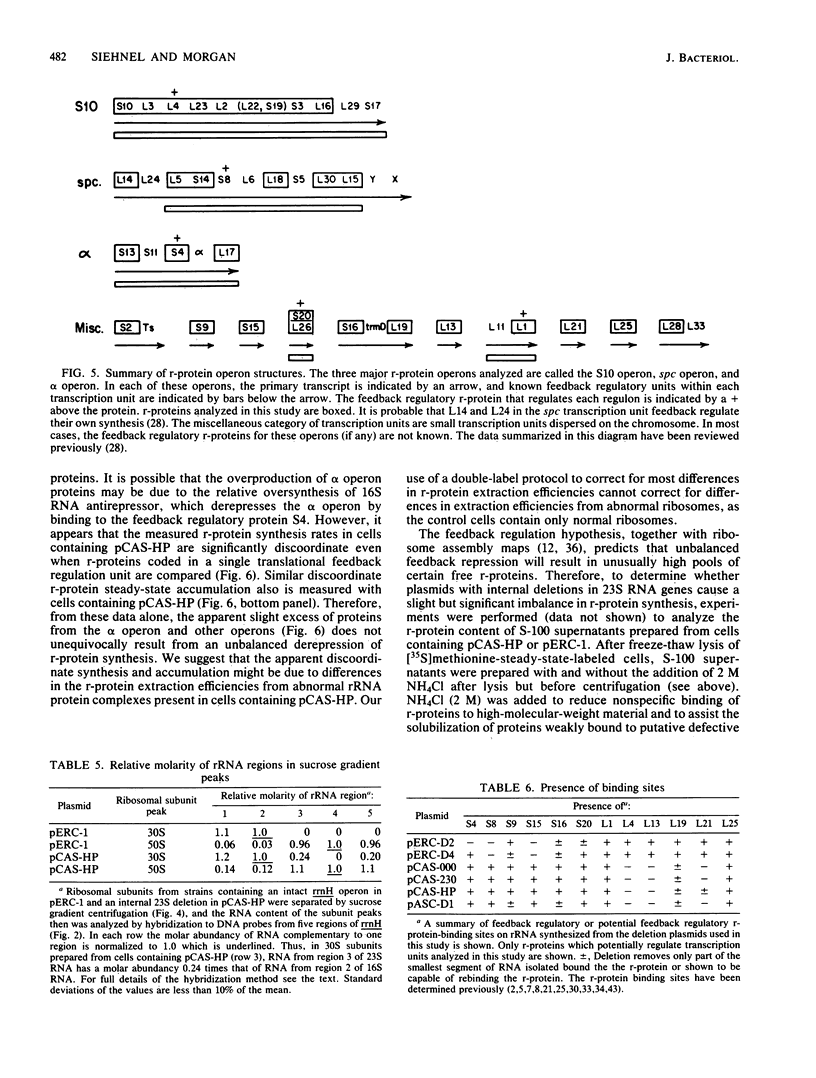
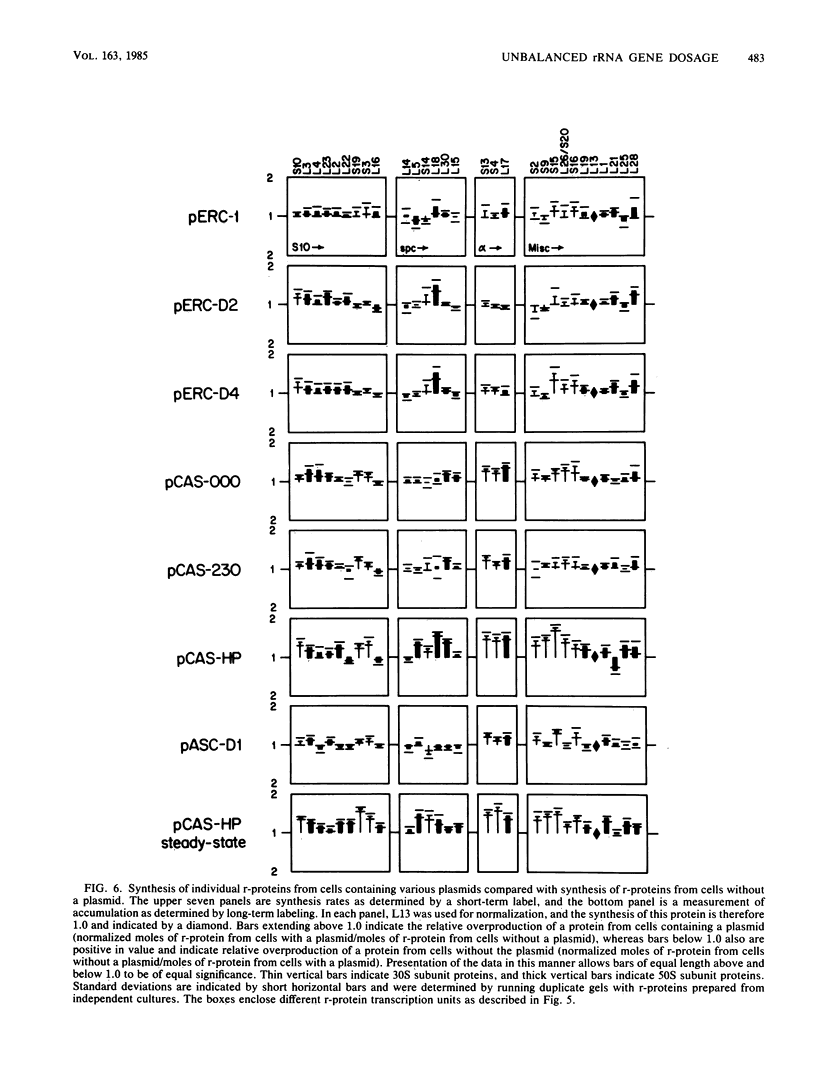
The synthesis of rRNA was unbalanced by the introduction of plasmids containing rRNA operons with large internal deletions. Significant unbalanced synthesis was achieved only when the deletions affected both 16S and 23S RNA genes or when the deletions affected the 23S RNA gene alone. Although large imbalances in rRNA synthesis resulted from deletions affecting 16S and 23S RNA genes or only 23S RNA genes, excess 16S RNA and defective rRNA species were rapidly degraded. Large imbalances in the synthesis of regions of rRNA did not result in significantly unbalanced synthesis of ribosomal proteins. It therefore is probable that excess intact 16S RNA is degraded because ribosomal proteins are not available for packaging the RNA into ribosomes. Defective RNA species also may be degraded for this reason or because proper ribosome assembly is prevented by the defects in RNA structure. We propose two possible explanations for the finding that unbalanced overproduction of binding sites for feedback ribosomal protein does not result in significant unbalanced translational feedback depression of ribosomal protein mRNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Hatfield G. W. Effects of promoter strengths and growth conditions on copy number of transcription-fusion vectors. J Biol Chem. 1984 Jun 25;259(12):7399–7403. [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt A., Ebel J. P. The secondary structure of the protein L1 binding region of ribosomal 23S RNA. Homologies with putative secondary structures of the L11 mRNA and of a region of mitochondrial 16S rRNA. Nucleic Acids Res. 1981 Jan 24;9(2):293–307. doi: 10.1093/nar/9.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen R. K., Duester G. L., Holmes W. M., Young J. M. Organization of transfer ribonucleic acid genes in the Escherichia coli chromosome. J Bacteriol. 1980 Dec;144(3):1083–1093. doi: 10.1128/jb.144.3.1083-1093.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesareni G., Muesing M. A., Polisky B. Control of ColE1 DNA replication: the rop gene product negatively affects transcription from the replication primer promoter. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6313–6317. doi: 10.1073/pnas.79.20.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Rodriguez R. L. Construction and characterization of the chloramphenicol-resistance gene cartridge: a new approach to the transcriptional mapping of extrachromosomal elements. Gene. 1982 Dec;20(2):305–316. doi: 10.1016/0378-1119(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Douthwaite S., Christensen A., Garrett R. A. Binding site of ribosomal proteins on prokaryotic 5S ribonucleic acids: a study with ribonucleases. Biochemistry. 1982 May 11;21(10):2313–2320. doi: 10.1021/bi00539a007. [DOI] [PubMed] [Google Scholar]
- Ehresmann B., Backendorf C., Ehresmann C., Ebel J. P. Characterization of the regions from E. coli 16 S RNA covalently linked to ribosomal proteins S4 and S20 after ultraviolet irradiation. FEBS Lett. 1977 Jun 15;78(2):261–266. doi: 10.1016/0014-5793(77)80319-0. [DOI] [PubMed] [Google Scholar]
- Gausing K. Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and of ribosomal protein messenger RNA at different growth rates. J Mol Biol. 1977 Sep 25;115(3):335–354. doi: 10.1016/0022-2836(77)90158-9. [DOI] [PubMed] [Google Scholar]
- Gausing K. Ribosomal protein in E. coli: rate of synthesis and pool size at different growth rates. Mol Gen Genet. 1974 Mar 6;129(1):61–75. doi: 10.1007/BF00269266. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Singh U. N. Biogenesis of ribosomes: free ribosomal protein pools in Escherichia coli. J Mol Biol. 1972 Aug 21;69(2):279–301. doi: 10.1016/0022-2836(72)90230-6. [DOI] [PubMed] [Google Scholar]
- Held W. A., Ballou B., Mizushima S., Nomura M. Assembly mapping of 30 S ribosomal proteins from Escherichia coli. Further studies. J Biol Chem. 1974 May 25;249(10):3103–3111. [PubMed] [Google Scholar]
- Ikemura T., Nomura M. Expression of spacer tRNA genes in ribosomal RNA transcription units carried by hybrid Col E1 plasmids in E. coli. Cell. 1977 Aug;11(4):779–793. doi: 10.1016/0092-8674(77)90291-4. [DOI] [PubMed] [Google Scholar]
- Jinks-Robertson S., Gourse R. L., Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983 Jul;33(3):865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- Jinks-Robertson S., Nomura M. Regulation of ribosomal protein synthesis in an Escherichia coli mutant missing ribosomal protein L1. J Bacteriol. 1981 Mar;145(3):1445–1447. doi: 10.1128/jb.145.3.1445-1447.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks-Robertson S., Nomura M. Ribosomal protein S4 acts in trans as a translational repressor to regulate expression of the alpha operon in Escherichia coli. J Bacteriol. 1982 Jul;151(1):193–202. doi: 10.1128/jb.151.1.193-202.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Lindahl L. Intermediates and time kinetics of the in vivo assembly of Escherichia coli ribosomes. J Mol Biol. 1975 Feb 15;92(1):15–37. doi: 10.1016/0022-2836(75)90089-3. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Operon-specific regulation of ribosomal protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6542–6546. doi: 10.1073/pnas.76.12.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R., Bremer H. Transcription of ribosomal component genes and lac in a relA+/relA pair of Escherichia coli strains. J Bacteriol. 1984 Sep;159(3):863–869. doi: 10.1128/jb.159.3.863-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A., Zimmermann R. A. RNA--protein interactions in the ribosome. IV. Structure and properties of binding sites for proteins S4, S16/S17 and S20 in the 16S RNA. J Mol Biol. 1978 May 5;121(1):17–39. doi: 10.1016/0022-2836(78)90260-7. [DOI] [PubMed] [Google Scholar]
- Mark L. G., Sigmund C. D., Morgan E. A. Spectinomycin resistance due to a mutation in an rRNA operon of Escherichia coli. J Bacteriol. 1983 Sep;155(3):989–994. doi: 10.1128/jb.155.3.989-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvaldi J., Pichon J., Delaage M., Marchis-Mouren G. Individual ribosomal protein pool size and turnover rate in Escherichia coli. J Mol Biol. 1974 Mar 25;84(1):83–96. doi: 10.1016/0022-2836(74)90213-7. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Müller R., Garrett R. A., Noller H. F. The structure of the RNA binding site of ribosomal proteins S8 and S15. J Biol Chem. 1979 May 25;254(10):3873–3878. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Belasco J. G., Cohen S. N., von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984 Nov 1;312(5989):75–77. doi: 10.1038/312075a0. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Nomura M., Traub P., Guthrie C., Nashimoto H. The assembly of ribosomes. J Cell Physiol. 1969 Oct;74(2 Suppl):241+–241+. doi: 10.1002/jcp.1040740428. [DOI] [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris T. E., Koch A. L. Effect of growth rate on the relative rates of synthesis of messenger, ribosomal and transfer RNA in Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):633–649. doi: 10.1016/0022-2836(72)90088-5. [DOI] [PubMed] [Google Scholar]
- Nowotny V., Nierhaus K. H. Initiator proteins for the assembly of the 50S subunit from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7238–7242. doi: 10.1073/pnas.79.23.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins P. O., Nomura M. Regulation of the S10 ribosomal protein operon in E. coli: nucleotide sequence at the start of the operon. Cell. 1981 Oct;26(2 Pt 2):205–211. doi: 10.1016/0092-8674(81)90303-2. [DOI] [PubMed] [Google Scholar]
- Olins P. O., Nomura M. Translational regulation by ribosomal protein S8 in Escherichia coli: structural homology between rRNA binding site and feedback target on mRNA. Nucleic Acids Res. 1981 Apr 10;9(7):1757–1764. doi: 10.1093/nar/9.7.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. III. Effects of rpsD mutations on expression of some ribosomal protein genes. Mol Gen Genet. 1979 Feb 1;169(3):271–278. doi: 10.1007/BF00382273. [DOI] [PubMed] [Google Scholar]
- Röhl R., Nierhaus K. H. Assembly map of the large subunit (50S) of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):729–733. doi: 10.1073/pnas.79.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells B. H., Davis F. C., Jr Biogenesis of 50 s particles in exponentially growing Escherichia coli. J Mol Biol. 1970 Jan 28;47(2):155–167. doi: 10.1016/0022-2836(70)90336-0. [DOI] [PubMed] [Google Scholar]
- Shen W. F., Squires C., Squires C. L. Nucleotide sequence of the rrnG ribosomal RNA promoter region of Escherichia coli. Nucleic Acids Res. 1982 May 25;10(10):3303–3313. doi: 10.1093/nar/10.10.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard H. M., Polisky B. Measurement of Plasmid copy number. Methods Enzymol. 1979;68:503–513. doi: 10.1016/0076-6879(79)68039-4. [DOI] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler G., Hasenbank R., Dabbs E. R. Expression of the L11-L1 operon in mutants of Escherichia coli lacking the ribosomal proteins L1 or L11. Mol Gen Genet. 1981;181(2):164–168. doi: 10.1007/BF00268422. [DOI] [PubMed] [Google Scholar]
- Yuki A., Brimacombe R. Nucleotide sequences of Escherichia coli 16-S RNA associated with ribosomal proteins S7, S9, S10, S14 and S19. Eur J Biochem. 1975 Aug 1;56(1):23–34. doi: 10.1111/j.1432-1033.1975.tb02203.x. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Archer R. H., Freedman L. P., Lindahl L. Role of attenuation in growth rate-dependent regulation of the S10 r-protein operon of E. coli. EMBO J. 1984 Jul;3(7):1561–1565. doi: 10.1002/j.1460-2075.1984.tb02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. M., Mueckl D., Lindahl L. Protein L4 of the E. coli ribosome regulates an eleven gene r protein operon. Cell. 1980 Sep;21(2):523–535. doi: 10.1016/0092-8674(80)90490-0. [DOI] [PubMed] [Google Scholar]