Abstract
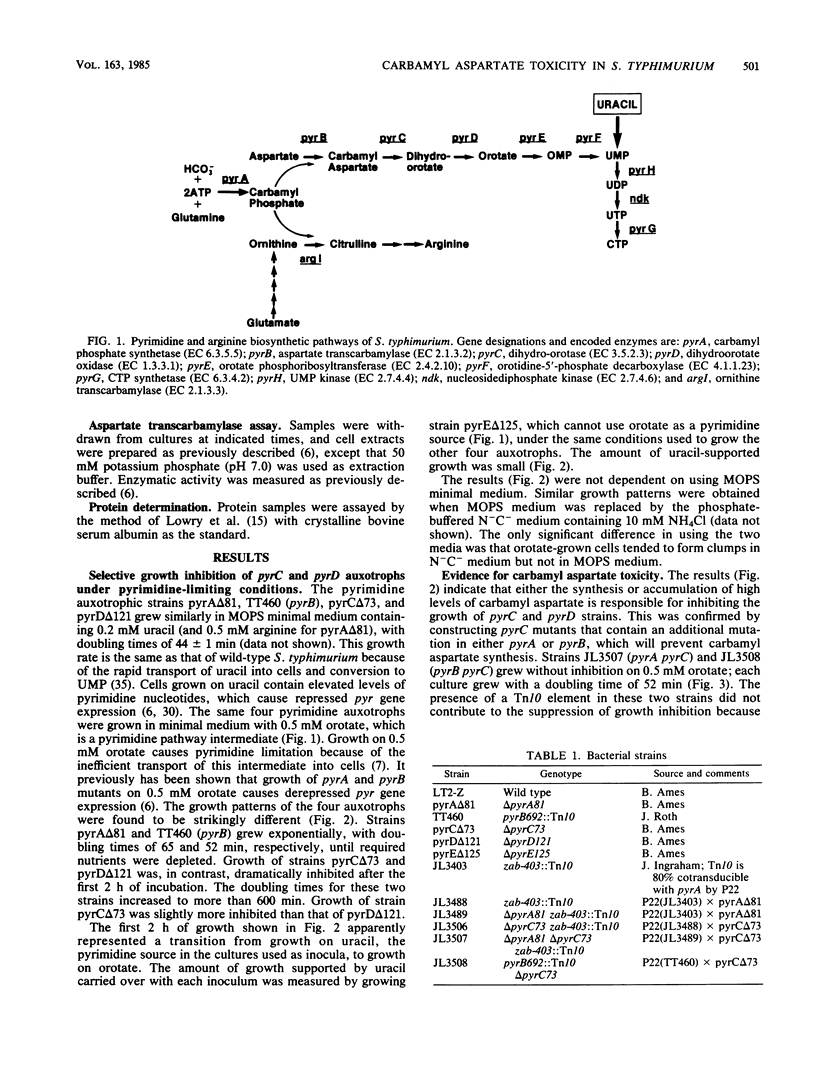
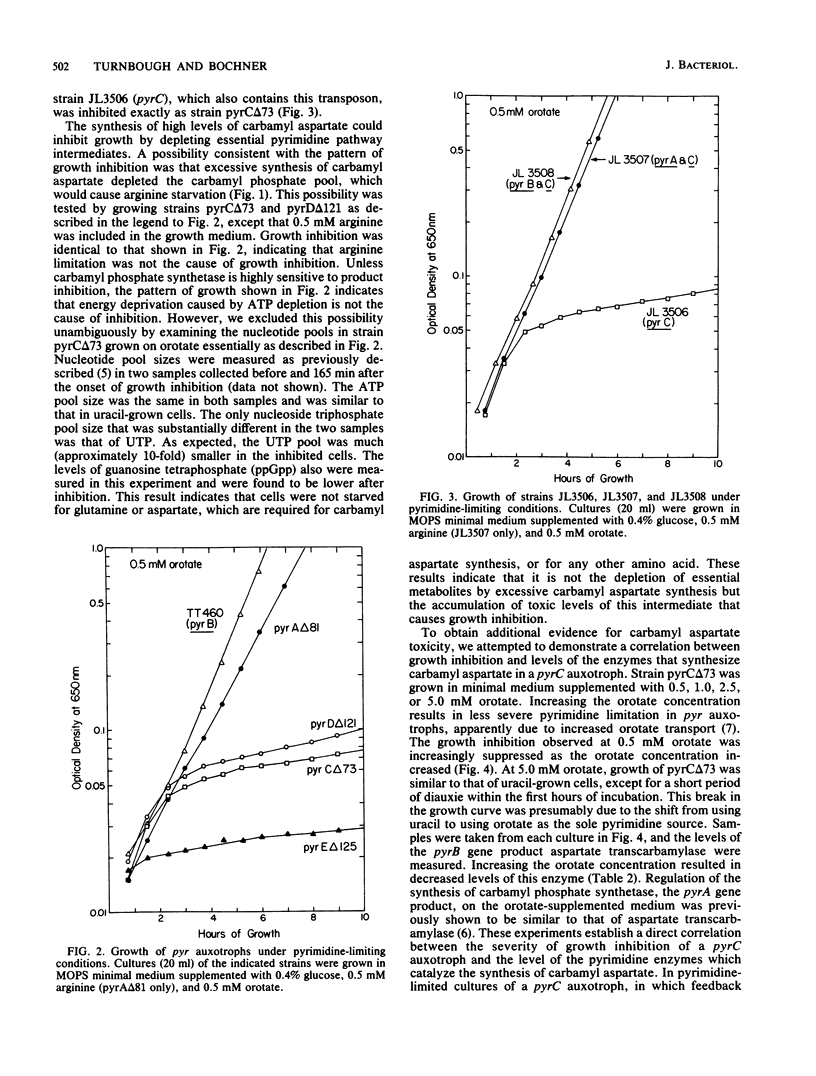
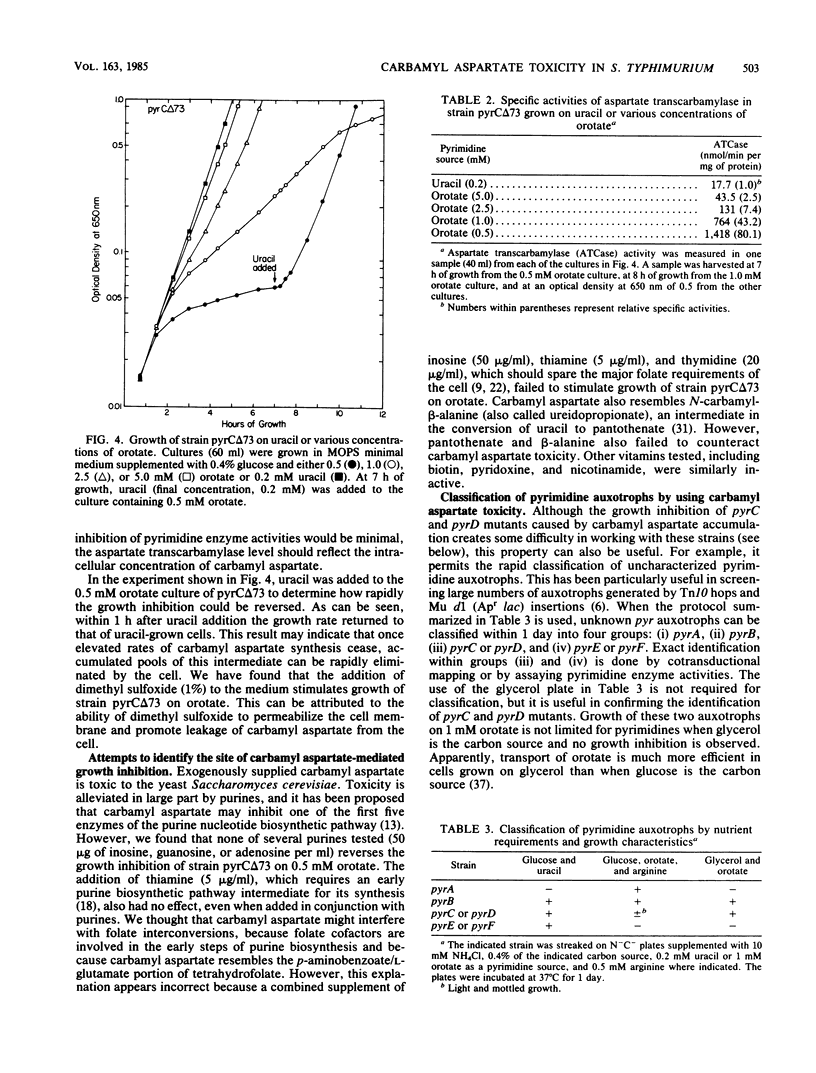
Growth of Salmonella typhimurium pyrC or pyrD auxotrophs was severely inhibited in media that caused derepressed pyr gene expression. No such inhibition was observed with derepressed pyrA and pyrB auxotrophs. Growth inhibition was not due to the depletion of essential pyrimidine biosynthetic pathway intermediates or substrates. This result and the pattern of inhibition indicated that the accumulation of the pyrimidine biosynthetic pathway intermediate carbamyl aspartate was toxic. This intermediate is synthesized by the sequential action of the first two enzymes of the pathway encoded by pyrA and pyrB and is a substrate for the pyrC gene product. It should accumulate to high levels in pyrC or pyrD mutants when expression of the pyrA and pyrB genes is elevated. The introduction of either a pyrA or pyrB mutation into a pyrC strain eliminated the observed growth inhibition. Additionally, a direct correlation was shown between the severity of growth inhibition of a pyrC auxotroph and the levels of the enzymes that synthesize carbamyl aspartate. The mechanism of carbamyl aspartate toxicity was not identified, but many potential sites of growth inhibition were excluded. Carbamyl aspartate toxicity was shown to be useful as a phenotypic trait for classifying pyrimidine auxotrophs and may also be useful for positive selection of pyrA or pyrB mutants. Finally, we discuss ways of overcoming growth inhibition of pyrC and pyrD mutants under derepressing conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Control of Escherichia coli carbamyl phosphate synthetase by purine and pyrimidine nucleotides. Biochemistry. 1966 Oct;5(10):3164–3169. doi: 10.1021/bi00874a013. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Csonka L. N., Howe M. M., Ingraham J. L., Pierson L. S., 3rd, Turnbough C. L., Jr Infection of Salmonella typhimurium with coliphage Mu d1 (Apr lac): construction of pyr::lac gene fusions. J Bacteriol. 1981 Jan;145(1):299–305. doi: 10.1128/jb.145.1.299-305.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Herman R. K. Pyrimidine pools and macromolecular composition of pyrimidine-limited Escherichia coli. J Bacteriol. 1970 Apr;102(1):118–123. doi: 10.1128/jb.102.1.118-123.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Greth M. L., Chevallier M. R., Lacroute F. Ureidosuccinic acid permeation in Saccharomyces cerevisiae. Biochim Biophys Acta. 1977 Feb 14;465(1):138–151. doi: 10.1016/0005-2736(77)90362-5. [DOI] [PubMed] [Google Scholar]
- Jenness D. D., Schachman H. K. pryB mutations as suppressors of arginine auxotrophy in Salmonella typhimurium. J Bacteriol. 1980 Jan;141(1):33–40. doi: 10.1128/jb.141.1.33-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelln R. A., O'Donovan G. A. Isolation and partial characterization of an argR mutant of Salmonella typhimurium. J Bacteriol. 1976 Nov;128(2):528–535. doi: 10.1128/jb.128.2.528-535.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C. T., Lacroute F., Exinger F. A regulatory interaction between pyrimidine and purine biosyntheses via ureidosuccinic acid. Mol Gen Genet. 1974;133(1):63–75. doi: 10.1007/BF00268678. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lissens W., Cunin R., Kelker N., Glansdorff N., Piérard A. In vitro synthesis of Escherichia coli carbamoylphosphate synthase: evidence for participation of the arginine repressor in cumulative repression. J Bacteriol. 1980 Jan;141(1):58–66. doi: 10.1128/jb.141.1.58-66.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navre M., Schachman H. K. Synthesis of aspartate transcarbamoylase in Escherichia coli: transcriptional regulation of the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1207–1211. doi: 10.1073/pnas.80.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. Biosynthesis of the pyrimidine moiety of thiamine. A new route of pyrimidine biosynthesis involving purine intermediates. Biochem J. 1968 Jan;106(1):279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan S. F., Kantrowitz E. R. Identification of a trans-acting regulatory factor involved in the control of the pyrimidine pathway in E. coli. Mol Gen Genet. 1983;192(1-2):264–271. doi: 10.1007/BF00327676. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Holoubek H., Gerhart J. C. Regulatory properties of intergeneric hybrids of aspartate transcarbamylase. Nat New Biol. 1972 Aug 30;238(87):264–266. doi: 10.1038/newbio238264a0. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Gigot D., Crabeel M., Halleux P., Thiry L. Repression of Escherichia coli carbamoylphosphate synthase: relationships with enzyme synthesis in the arginine and pyrimidine pathways. J Bacteriol. 1976 Jul;127(1):291–301. doi: 10.1128/jb.127.1.291-301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Yashphe J. Mutations affecting uridine monophosphate pyrophosphorylase or the argR gene in Escherichia coli. Effects on carbamoyl phosphate and pyrimidine biosynthesis and on uracil uptake. Mol Gen Genet. 1972;118(3):235–245. doi: 10.1007/BF00333460. [DOI] [PubMed] [Google Scholar]
- Piérard A., Wiame J. M. Regulation and mutation affecting a glutamine dependent formation of carbamyl phosphate in Escherichia coli. Biochem Biophys Res Commun. 1964 Feb 18;15(1):76–81. doi: 10.1016/0006-291x(64)90106-8. [DOI] [PubMed] [Google Scholar]
- Poulsen P., Bonekamp F., Jensen K. F. Structure of the Escherichia coli pyrE operon and control of pyrE expression by a UTP modulated intercistronic attentuation. EMBO J. 1984 Aug;3(8):1783–1790. doi: 10.1002/j.1460-2075.1984.tb02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P., Jensen K. F., Valentin-Hansen P., Carlsson P., Lundberg L. G. Nucleotide sequence of the Escherichia coli pyrE gene and of the DNA in front of the protein-coding region. Eur J Biochem. 1983 Sep 15;135(2):223–229. doi: 10.1111/j.1432-1033.1983.tb07641.x. [DOI] [PubMed] [Google Scholar]
- Roof W. D., Foltermann K. F., Wild J. R. The organization and regulation of the pyrBI operon in E. coli includes a rho-independent attenuator sequence. Mol Gen Genet. 1982;187(3):391–400. doi: 10.1007/BF00332617. [DOI] [PubMed] [Google Scholar]
- SLOTNICK I. J., WEINFELD H. Dihydrouracil as a growth factor for mutant strains of Escherichia coli. J Bacteriol. 1957 Aug;74(2):122–125. doi: 10.1128/jb.74.2.122-125.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Neuhard J. Control of expression of the pyr genes in Salmonella typhimurium: effects of variations in uridine and cytidine nucleotide pools. J Bacteriol. 1975 Mar;121(3):814–822. doi: 10.1128/jb.121.3.814-822.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for catalytic and regulatory subunits of aspartate transcarbamylase. J Mol Biol. 1973 May 25;76(3):363–378. doi: 10.1016/0022-2836(73)90510-x. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Hicks K. L., Donahue J. P. Attenuation control of pyrBI operon expression in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 Jan;80(2):368–372. doi: 10.1073/pnas.80.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr Regulation of Escherichia coli aspartate transcarbamylase synthesis by guanosine tetraphosphate and pyrimidine ribonucleoside triphosphates. J Bacteriol. 1983 Feb;153(2):998–1007. doi: 10.1128/jb.153.2.998-1007.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Lee C. E., Wild J. R. Genetic and biochemical characterization of distinct transport systems for uracil, uridine and cytidine in Salmonella typhimurium. Mol Gen Genet. 1980 Apr;178(1):121–130. doi: 10.1007/BF00267220. [DOI] [PubMed] [Google Scholar]
- Womack J. E., O'Donovan G. A. Orotic acid excretion in some wild-type strains of Escherichia coli K-12. J Bacteriol. 1978 Nov;136(2):825–827. doi: 10.1128/jb.136.2.825-827.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak V. L., Kelln R. A. 5-Fluoroorotate-resistant mutants of Salmonella typhimurium. Can J Microbiol. 1978 Nov;24(11):1339–1345. doi: 10.1139/m78-216. [DOI] [PubMed] [Google Scholar]
- Zak V. L., Kelln R. A. A Salmonella typhimurium mutant dependent upon carbamyl aspartate for resistance to 5-fluorouracil is specifically affected in ubiquinone biosynthesis. J Bacteriol. 1981 Feb;145(2):1095–1098. doi: 10.1128/jb.145.2.1095-1098.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


