Abstract
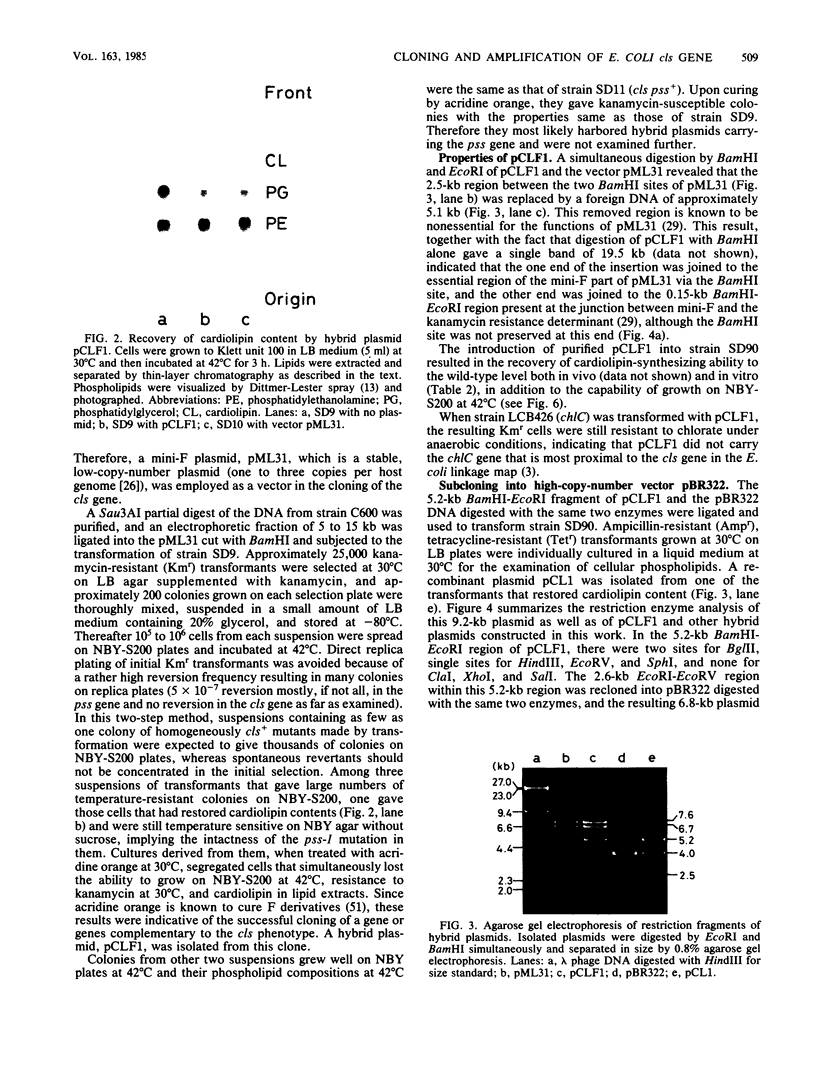
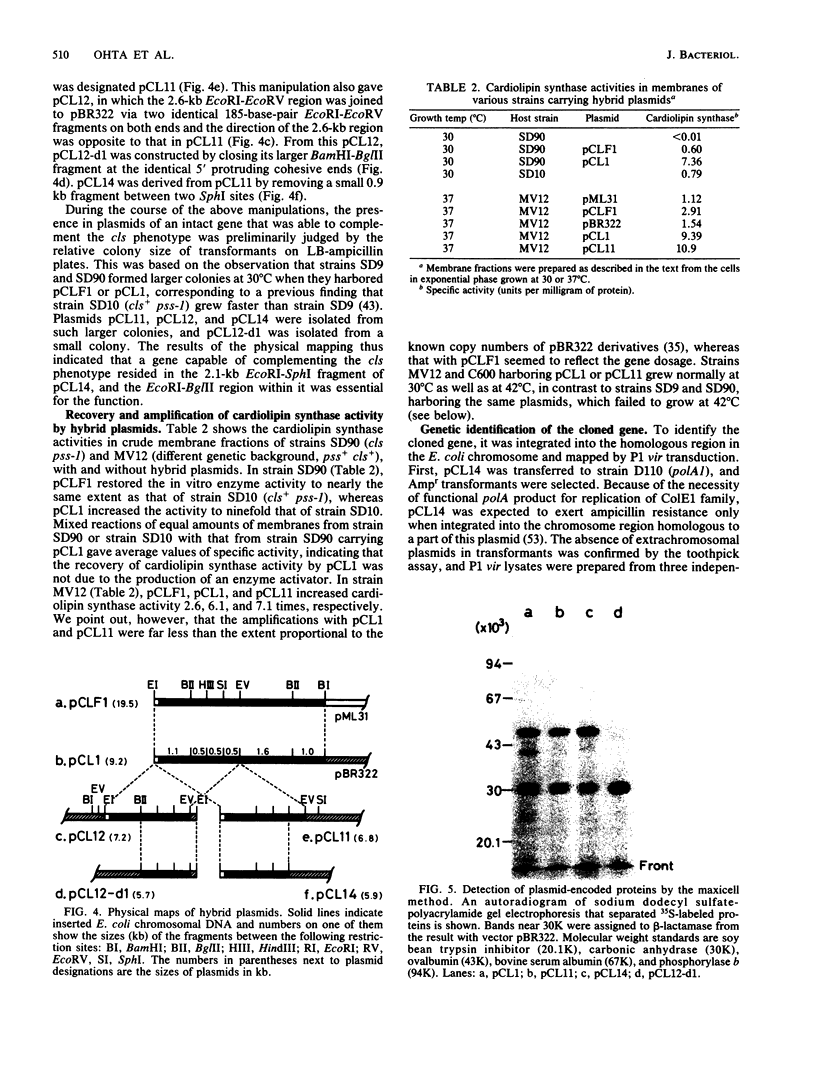
The cls gene responsible for cardiolipin synthesis in Escherichia coli K-12 was cloned in a 5-kilobase-pair DNA fragment inserted in a mini-F vector, pML31, and then subcloned into a 2.0-kilobase-pair fragment inserted in pBR322. The initial selection of the gene was accomplished in a cls pss-1 double mutant that had lesions in both cardiolipin and phosphatidylserine synthases and required either the cls or the pss gene product for normal growth at 42 degrees C in a broth medium, NBY, supplemented with 200 mM sucrose. The cloned gene was identified as the cls gene by the recovery and amplification of both cardiolipin and cardiolipin synthase in a cls mutant as well as by the integration of a pBR322 derivative into its genetic locus at 27 min on the chromosome of a polA1 mutant. The maxicell analysis indicated that a protein of molecular weight 46,000 is the gene product. The cls gene is thus most likely the structural gene coding for cardiolipin synthase. Hybrid plasmids of high copy numbers containing the cls gene were growth inhibitory to pss-I mutants under the above selective conditions, whereas they inhibited neither the growth of pss-I mutants at 30 degrees C nor that of pss+ strains at any temperature. Amplification of cardiolipin synthase activity was observed, but was not proportional to the probable gene dosage (the enzyme activity was at most 10 times that in wild-type cells), and cardiolipin synthesis in vivo was at the maximum 1.5 times that in wild-type strains, implying the presence in E. coli cells of a mechanism that avoids cardiolipin overproduction, which is possibly disadvantageous to proper membrane functions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Cronan J. E., Jr Mutants of Escherichia coli defective in membrane phospholipid synthesis. Phenotypic suppression of sn-glycerol-3-phosphate acyltransferase Km mutants by loss of feedback inhibition of the biosynthetic sn-glycerol-3-phosphate dehydrogenase. J Biol Chem. 1975 Sep 25;250(18):7153–7158. [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P. K., Wu H. C. Biosynthesis of the covalently linked diglyceride in murein lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5318–5322. doi: 10.1073/pnas.74.12.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Guest J. R. Biochemical and genetic studies with nitrate reductase C-gene mutants of Escherichia coli. Mol Gen Genet. 1969;105(4):285–297. doi: 10.1007/BF00277583. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg C. B., Kennedy E. P. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):648–651. doi: 10.1073/pnas.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. W., Engel R., Tropp B. E. Correlation of 3,4-dihydroxybutyl 1-phosphonate resistance with a defect in cardiolipin synthesis in Escherichia coli. J Bacteriol. 1984 Mar;157(3):846–856. doi: 10.1128/jb.157.3.846-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B. J., Kennedy E. P. The biosynthesis of membrane-derived oligosaccharides. A membrane-bound phosphoglycerol transferase. J Biol Chem. 1983 Feb 25;258(4):2394–2398. [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Kung H. F., Henning U. Limiting availability of binding sites for dehydrogenases on the cell membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):925–929. doi: 10.1073/pnas.69.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Hirabayshi T., Dowhan W. Phosphatidylglycerol biosynthesis in Bacillus licheniformis Resolution of membrane-bound enzymes by affinity chromatography on cytidinediphospho-sn-1,2-diacylglycerol Sepharose. Biochemistry. 1976 Mar 9;15(5):974–979. doi: 10.1021/bi00650a005. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. Restriction endonuclease mapping and mutagenesis of the F sex factor replication region. Mol Gen Genet. 1977 Apr 29;152(3):175–182. doi: 10.1007/BF00268815. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A., Shibuya I. Membrane phospholipid synthesis and phenotypic correlation of an Escherichia coli pss mutant. J Bacteriol. 1977 Nov;132(2):434–443. doi: 10.1128/jb.132.2.434-443.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A., Waggoner K., Louie K., Dowhan W. Cloning of genes involved in membrane lipid synthesis. Effects of amplification of phosphatidylserine synthase in Escherichia coli. J Biol Chem. 1981 Mar 10;256(5):2219–2225. [PubMed] [Google Scholar]
- Ota A., Shibuya I., Maruo B., Ishinaga M., Kito M. An extremely labile phosphatidylserine synthetase of an Escherichia coli mutant with the temperature-sensitive formation of phosphatidylethanolamine. Biochim Biophys Acta. 1974 Jun 26;348(3):449–454. [PubMed] [Google Scholar]
- Pluschke G., Hirota Y., Overath P. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J Biol Chem. 1978 Jul 25;253(14):5048–5055. [PubMed] [Google Scholar]
- Pluschke G., Overath P. Function of phospholipids in Escherichia coli. Influence of changes in polar head group composition on the lipid phase transition and characterization of a mutant containing only saturated phospholipid acyl chains. J Biol Chem. 1981 Apr 10;256(7):3207–3212. [PubMed] [Google Scholar]
- Puig J., Azoulay E., Pichinoty F., Gendre J. Genetic mapping of the chl C gene of the nitrate reductase A system in Escherichia coli K12. Biochem Biophys Res Commun. 1969 Jun 6;35(5):659–662. doi: 10.1016/0006-291x(69)90455-0. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. Function of cytidine diphosphate-diglyceride and deoxycytidine diphosphate-diglyceride in the biogenesis of membrane lipids in Escherichia coli. J Biol Chem. 1973 Feb 10;248(3):1098–1105. [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya I., Miyazaki C., Ohta A. Alteration of phospholipid composition by combined defects in phosphatidylserine and cardiolipin synthases and physiological consequences in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1086–1092. doi: 10.1128/jb.161.3.1086-1092.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya I., Yamagoe S., Miyazaki C., Matsuzaki H., Ohta A. Biosynthesis of novel acidic phospholipid analogs in Escherichia coli. J Bacteriol. 1985 Feb;161(2):473–477. doi: 10.1128/jb.161.2.473-477.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider M. D. Control of membrane lipid synthesis in Escherichia coli during growth and during the stringent response. J Biol Chem. 1979 Aug 10;254(15):7917–7202. [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Tunaitis E., Cronan J. E., Jr Characterization of the cardiolipin synthetase activity of Escherichia coli envelopes. Arch Biochem Biophys. 1973 Apr;155(2):420–427. doi: 10.1016/0003-9861(73)90132-x. [DOI] [PubMed] [Google Scholar]
- Tyhach R. J., Hawrot E., Satre M., Kennedy E. P. Increased synthesis of phosphatidylserine decarboxylase in a strain of Escherichia coli bearing a hybrid plasmid. Altered association of enzyme with the membrane. J Biol Chem. 1979 Feb 10;254(3):627–633. [PubMed] [Google Scholar]
- Vik S. B., Georgevich G., Capaldi R. A. Diphosphatidylglycerol is required for optimal activity of beef heart cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1456–1460. doi: 10.1073/pnas.78.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J., Kline B. C. Mutation and identification of the F plasmid locus determining resistance to acridine orange curing. Plasmid. 1980 Nov;4(3):276–280. doi: 10.1016/0147-619x(80)90066-9. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Tomizawa J. Establishment of Escherichia coli cells with an integrated high copy number plasmid. Mol Gen Genet. 1980;178(3):525–533. doi: 10.1007/BF00337857. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]